Recombination activity of human recombination-activating gene 2 (RAG2) mutations and correlation with clinical phenotype
- PMID: 29772310
- PMCID: PMC6295349
- DOI: 10.1016/j.jaci.2018.04.027
Recombination activity of human recombination-activating gene 2 (RAG2) mutations and correlation with clinical phenotype
Abstract
Background: Mutations in recombination-activating gene (RAG) 1 and RAG2 are associated with a broad range of clinical and immunologic phenotypes in human subjects.
Objective: Using a flow cytometry-based assay, we aimed to measure the recombinase activity of naturally occurring RAG2 mutant proteins and to correlate our results with the severity of the clinical and immunologic phenotype.
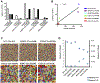
Methods: Abelson virus-transformed Rag2-/- pro-B cells engineered to contain an inverted green fluorescent protein (GFP) cassette flanked by recombination signal sequences were transduced with retroviruses encoding either wild-type or 41 naturally occurring RAG2 variants. Bicistronic vectors were used to introduce compound heterozygous RAG2 variants. The percentage of GFP-expressing cells was evaluated by using flow cytometry, and high-throughput sequencing was used to analyze rearrangements at the endogenous immunoglobulin heavy chain (Igh) locus.
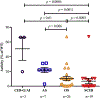
Results: The RAG2 variants showed a wide range of recombination activity. Mutations associated with severe combined immunodeficiency and Omenn syndrome had significantly lower activity than those detected in patients with less severe clinical presentations. Four variants (P253R, F386L, N474S, and M502V) previously thought to be pathogenic were found to have wild-type levels of activity. Use of bicistronic vectors permitted us to assess more carefully the effect of compound heterozygous mutations, with good correlation between GFP expression and the number and diversity of Igh rearrangements.
Conclusions: Our data support genotype-phenotype correlation in the setting of RAG2 deficiency. The assay described can be used to define the possible disease-causing role of novel RAG2 variants and might help predict the severity of the clinical phenotype.
Keywords: Omenn syndrome; Recombination-activating gene 2; VDJ recombination; autoimmunity; genotype-phenotype correlation; severe combined immunodeficiency.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: The authors declare that they have relevant no conflicts of interest.
Figures
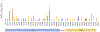


References
-
- Schwarz K, Gauss GH, Ludwig L, Pannicke U, Li Z, Lindner D, et al. RAG mutations in human B cell-negative SCID. Science 1996;274:97–9. - PubMed
-
- Villa A, Sobacchi C, Vezzoni P. Omenn syndrome in the context of other B cell-negative severe combined immunodeficiencies. Isr Med Assoc J 2002;4:218–21. - PubMed
-
- Villa A, Sobacchi C, Notarangelo LD, Bozzi F, Abinun M, Abrahamsen T, et al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood 2001;97:81–8. - PubMed
-
- Schuetz C, Huck K, Gudowius S, Megahed M, Feyen O, Hubner B, et al. An immunodeficiency disease with RAG mutations and granulomas. N Engl J Med 2008; 358:2030–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

