Evaluation of two prognostic indices for adult T-cell leukemia/lymphoma in the subtropical endemic area, Okinawa, Japan
- PMID: 29772611
- PMCID: PMC6029833
- DOI: 10.1111/cas.13641
Evaluation of two prognostic indices for adult T-cell leukemia/lymphoma in the subtropical endemic area, Okinawa, Japan
Abstract
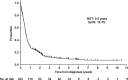
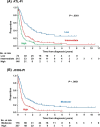
Aggressive adult T-cell leukemia/lymphoma (ATL) has an extremely poor prognosis and is hyperendemic in Okinawa, Japan. This study evaluated two prognostic indices (PIs) for aggressive ATL, the ATL-PI and Japan Clinical Oncology Group (JCOG)-PI, in a cohort from Okinawa. The PIs were originally developed using two different Japanese cohorts that included few patients from Okinawa. The endpoint was overall survival (OS). Multivariable Cox regression analyses in the cohort of 433 patients revealed that all seven factors for calculating each PI were statistically significant prognostic predictors. Three-year OS rates for ATL-PI were 35.9% (low-risk, n = 66), 10.4% (intermediate-risk, n = 256), and 1.6% (high-risk, n = 111), and those for JCOG-PI were 22.4% (moderate-risk, n = 176) and 5.3% (high-risk, n = 257). The JCOG-PI moderate-risk group included both the ATL-PI low- and intermediate-risk groups. ATL-PI more clearly identified the low-risk patient subgroup than JCOG-PI. To evaluate the external validity of the two PIs, we also assessed prognostic discriminability among 159 patients who loosely met the eligibility criteria of a previous clinical trial. Three-year OS rates for ATL-PI were 34.5% (low-risk, n = 42), 9.2% (intermediate-risk, n = 109), and 12.5% (high-risk, n = 8). Those for JCOG-PI were 22.4% (moderate-risk, n = 95) and 7.6% (high-risk, n = 64). The low-risk ATL-PI group had a better prognosis than the JCOG-PI moderate-risk group, suggesting that ATL-PI would be more useful than JCOG-PI for establishing and examining novel treatment strategies for ATL patients with a better prognosis. In addition, strongyloidiasis, previously suggested to be associated with ATL-related deaths in Okinawa, was not a prognostic factor in this study.
Keywords: ATL-PI; JCOG-PI; Okinawa; adult T-cell leukemia/lymphoma; strongyloidiasis.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
Japan Clinical Oncology Group (JCOG) prognostic index and characterization of long-term survivors of aggressive adult T-cell leukaemia-lymphoma (JCOG0902A).Br J Haematol. 2014 Sep;166(5):739-48. doi: 10.1111/bjh.12962. Epub 2014 Jun 14. Br J Haematol. 2014. PMID: 24931507
-
Characterization of patients with aggressive adult T-cell leukemia-lymphoma in Okinawa, Japan: a retrospective analysis of a large cohort.Int J Hematol. 2016 Oct;104(4):468-75. doi: 10.1007/s12185-016-2042-y. Epub 2016 Jun 21. Int J Hematol. 2016. PMID: 27329124
-
Human T-cell leukemia virus type I Tax genotype analysis in Okinawa, the southernmost and remotest islands of Japan: Different distributions compared with mainland Japan and the potential value for the prognosis of aggressive adult T-cell leukemia/lymphoma.Leuk Res. 2017 Oct;61:18-24. doi: 10.1016/j.leukres.2017.08.006. Epub 2017 Aug 18. Leuk Res. 2017. PMID: 28866351
-
Human T lymphotropic virus type-I and adult T-cell leukemia in Japan.Int J Hematol. 2002 Aug;76 Suppl 2:240-5. doi: 10.1007/BF03165123. Int J Hematol. 2002. PMID: 12430931 Review.
-
Lymphoma study group of JCOG.Jpn J Clin Oncol. 2012 Feb;42(2):85-95. doi: 10.1093/jjco/hyr168. Epub 2011 Dec 6. Jpn J Clin Oncol. 2012. PMID: 22147803 Review.
Cited by
-
Clinical characterization and prognosis of T cell acute lymphoblastic leukemia with high CRLF2 gene expression in children.PLoS One. 2019 Dec 12;14(12):e0224652. doi: 10.1371/journal.pone.0224652. eCollection 2019. PLoS One. 2019. PMID: 31830053 Free PMC article.
-
Prognostic indices for peripheral T-cell lymphoma - not otherwise specified and adult T-cell leukemia/lymphoma: From past to future.J Clin Exp Hematop. 2023 Mar 28;63(1):1-11. doi: 10.3960/jslrt.22034. Epub 2023 Jan 30. J Clin Exp Hematop. 2023. PMID: 36709976 Free PMC article.
References
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481‐492. - PubMed
-
- Miyoshi I, Kubonishi I, Yoshimoto S, et al. Type C virus particles in a cord T‐cell line derived by co‐cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770‐771. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

