Paradoxical Effect of Chloroquine Treatment in Enhancing Chikungunya Virus Infection
- PMID: 29772762
- PMCID: PMC5977261
- DOI: 10.3390/v10050268
Paradoxical Effect of Chloroquine Treatment in Enhancing Chikungunya Virus Infection
Abstract
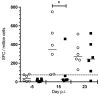
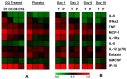
Since 2005, Chikungunya virus (CHIKV) re-emerged and caused numerous outbreaks in the world, and finally, was introduced into the Americas in 2013. The lack of CHIKV-specific therapies has led to the use of non-specific drugs. Chloroquine, which is commonly used to treat febrile illnesses in the tropics, has been shown to inhibit CHIKV replication in vitro. To assess the in vivo effect of chloroquine, two complementary studies were performed: (i) a prophylactic study in a non-human primate model (NHP); and (ii) a curative study "CuraChik", which was performed during the Reunion Island outbreak in 2006 in a human cohort. Clinical, biological, and immunological data were compared between treated and placebo groups. Acute CHIKV infection was exacerbated in NHPs treated with prophylactic administration of chloroquine. These NHPs displayed a higher viremia and slower viral clearance (p < 0.003). Magnitude of viremia was correlated to the type I IFN response (Rho = 0.8, p < 0.001) and severe lymphopenia (Rho = 0.8, p < 0.0001), while treatment led to a delay in both CHIKV-specific cellular and IgM responses (p < 0.02 and p = 0.04, respectively). In humans, chloroquine treatment did not affect viremia or clinical parameters during the acute stage of the disease (D1 to D14), but affected the levels of C-reactive Protein (CRP), IFNα, IL-6, and MCP1 over time (D1 to D16). Importantly, no positive effect could be detected on prevalence of persistent arthralgia at Day 300. Although inhibitory in vitro, chloroquine as a prophylactic treatment in NHPs enhances CHIKV replication and delays cellular and humoral response. In patients, curative chloroquine treatment during the acute phase decreases the levels of key cytokines, and thus may delay adaptive immune responses, as observed in NHPs, without any suppressive effect on peripheral viral load.
Keywords: alphavirus; chikungunya; chloroquine; macaque; monocyte-macrophage.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
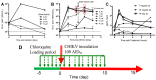
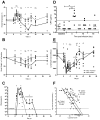
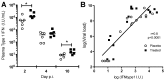
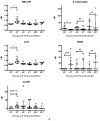

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

