Selected Plant Metabolites Involved in Oxidation-Reduction Processes during Bud Dormancy and Ontogenetic Development in Sweet Cherry Buds (Prunus avium L.)
- PMID: 29772774
- PMCID: PMC6099681
- DOI: 10.3390/molecules23051197
Selected Plant Metabolites Involved in Oxidation-Reduction Processes during Bud Dormancy and Ontogenetic Development in Sweet Cherry Buds (Prunus avium L.)
Abstract
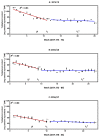
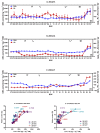
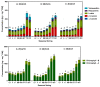
Many biochemical processes are involved in regulating the consecutive transition of different phases of dormancy in sweet cherry buds. An evaluation based on a metabolic approach has, as yet, only been partly addressed. The aim of this work, therefore, was to determine which plant metabolites could serve as biomarkers for the different transitions in sweet cherry buds. The focus here was on those metabolites involved in oxidation-reduction processes during bud dormancy, as determined by targeted and untargeted mass spectrometry-based methods. The metabolites addressed included phenolic compounds, ascorbate/dehydroascorbate, reducing sugars, carotenoids and chlorophylls. The results demonstrate that the content of phenolic compounds decrease until the end of endodormancy. After a long period of constancy until the end of ecodormancy, a final phase of further decrease followed up to the phenophase open cluster. The main phenolic compounds were caffeoylquinic acids, coumaroylquinic acids and catechins, as well as quercetin and kaempferol derivatives. The data also support the protective role of ascorbate and glutathione in the para- and endodormancy phases. Consistent trends in the content of reducing sugars can be elucidated for the different phenophases of dormancy, too. The untargeted approach with principle component analysis (PCA) clearly differentiates the different timings of dormancy giving further valuable information.
Keywords: Prunus avium L.; anti-oxidative capacity; ascorbate; dormancy; flower buds; phenolics; redox-metabolites.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures



References
-
- Chmielewski F.M., Gotz K.P., Homann T., Huschek G., Rawel H.M. Identification of endodormancy release for cherries (Prunus Avium L.) by abscisic acid and sugars. J. Hortic. 2017;4:210.
-
- Chmielewski F.M., Gotz K.P. Identification and timing of dormant and ontogenetic phase for sweet cherries in northeast Germany for modelling purposes. J. Hortic. 2017;4:205.
-
- Takemura Y., Kuroki K., Jiang M.F., Matsumoto K., Tamura F. Identification of the expressed protein and the impact of change in ascorbate peroxidase activity related to endodormancy breaking in Pyrus pyrifolia. Plant Physiol. Biochem. 2015;86:121–129. doi: 10.1016/j.plaphy.2014.11.016. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

