Oxidative Stress in Preeclampsia and Placental Diseases
- PMID: 29772777
- PMCID: PMC5983711
- DOI: 10.3390/ijms19051496
Oxidative Stress in Preeclampsia and Placental Diseases
Abstract
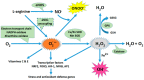
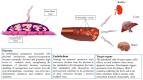
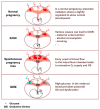
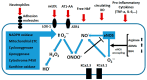
Preeclampsia is a persistent hypertensive gestational disease characterized by high blood pressure and proteinuria, which presents from the second trimester of pregnancy. At the cellular level, preeclampsia has largely been associated with the release of free radicals by the placenta. Placenta-borne oxidative and nitrosative stresses are even sometimes considered as the major molecular determinants of the maternal disease. In this review, we present the recent literature evaluating free radical production in both normal and pathological placentas (including preeclampsia and other major pregnancy diseases), in humans and animal models. We then assess the putative effects of these free radicals on the placenta and maternal endothelium. This analysis was conducted with regard to recent papers and possible therapeutic avenues.
Keywords: oxidative stress; placenta; preeclampsia; pregnancy; vascular endothelium.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
References
-
- Zabul P., Wozniak M., Slominski A.T., Preis K., Gorska M., Korozan M., Wieruszewski J., Zmijewski M.A., Zabul E., Tuckey R., et al. A proposed molecular mechanism of high-dose vitamin d3 supplementation in prevention and treatment of preeclampsia. Int. J. Mol. Sci. 2015;16:13043–13064. doi: 10.3390/ijms160613043. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

