Tau Fibril Formation in Cultured Cells Compatible with a Mouse Model of Tauopathy
- PMID: 29772786
- PMCID: PMC5983680
- DOI: 10.3390/ijms19051497
Tau Fibril Formation in Cultured Cells Compatible with a Mouse Model of Tauopathy
Abstract
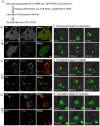
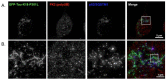
Neurofibrillary tangles composed of hyperphosphorylated tau protein are primarily neuropathological features of a number of neurodegenerative diseases collectively termed tauopathy. To understand the mechanisms underlying the cause of tauopathy, precise cellular and animal models are required. Recent data suggest that the transient introduction of exogenous tau can accelerate the development of tauopathy in the brains of non-transgenic and transgenic mice expressing wild-type human tau. However, the transmission mechanism leading to tauopathy is not fully understood. In this study, we developed cultured-cell models of tauopathy representing a human tauopathy. Neuro2a (N2a) cells containing propagative tau filaments were generated by introducing purified tau fibrils. These cell lines expressed full-length (2N4R) human tau and the green fluorescent protein (GFP)-fused repeat domain of tau with P301L mutation. Immunocytochemistry and super-resolution microscopic imaging revealed that tau inclusions exhibited filamentous morphology and were composed of both full-length and repeat domain fragment tau. Live-cell imaging analysis revealed that filamentous tau inclusions are transmitted to daughter cells, resulting in yeast-prion-like propagation. By a standard method of tau preparation, both full-length tau and repeat domain fragments were recovered in sarkosyl insoluble fraction. Hyperphosphorylation of full-length tau was confirmed by the immunoreactivity of phospho-Tau antibodies and mobility shifts by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). These properties were similar to the biochemical features of P301L mutated human tau in a mouse model of tauopathy. In addition, filamentous tau aggregates in cells barely co-localized with ubiquitins, suggesting that most tau aggregates were excluded from protein degradation systems, and thus propagated to daughter cells. The present cellular model of tauopathy will provide an advantage for dissecting the mechanisms of tau aggregation and degradation and be a powerful tool for drug screening to prevent tauopathy.
Keywords: cellular model; sarkosyl insoluble tau; super-resolution microscopy; tauopathy.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures





Similar articles
-
Human tauopathy-derived tau strains determine the substrates recruited for templated amplification.Brain. 2021 Sep 4;144(8):2333-2348. doi: 10.1093/brain/awab091. Brain. 2021. PMID: 33693528 Free PMC article.
-
GFP-Mutant Human Tau Transgenic Mice Develop Tauopathy Following CNS Injections of Alzheimer's Brain-Derived Pathological Tau or Synthetic Mutant Human Tau Fibrils.J Neurosci. 2017 Nov 22;37(47):11485-11494. doi: 10.1523/JNEUROSCI.2393-17.2017. Epub 2017 Oct 6. J Neurosci. 2017. PMID: 28986461 Free PMC article.
-
'Prion-like' propagation of mouse and human tau aggregates in an inducible mouse model of tauopathy.Neurodegener Dis. 2010;7(1-3):28-31. doi: 10.1159/000283479. Epub 2010 Feb 13. Neurodegener Dis. 2010. PMID: 20160454
-
[Neuropathology of tauopathy].Brain Nerve. 2013 Dec;65(12):1445-58. Brain Nerve. 2013. PMID: 24323931 Review. Japanese.
-
The ordered assembly of tau is the gain-of-toxic function that causes human tauopathies.Alzheimers Dement. 2016 Oct;12(10):1040-1050. doi: 10.1016/j.jalz.2016.09.001. Epub 2016 Sep 26. Alzheimers Dement. 2016. PMID: 27686274 Review.
Cited by
-
A next-generation iPSC-derived forebrain organoid model of tauopathy with tau fibrils by AAV-mediated gene transfer.Cell Rep Methods. 2022 Sep 8;2(9):100289. doi: 10.1016/j.crmeth.2022.100289. eCollection 2022 Sep 19. Cell Rep Methods. 2022. PMID: 36160042 Free PMC article.
-
Populations of Tau Conformers Drive Prion-like Strain Effects in Alzheimer's Disease and Related Dementias.Cells. 2022 Sep 26;11(19):2997. doi: 10.3390/cells11192997. Cells. 2022. PMID: 36230957 Free PMC article. Review.
-
Nasal Extracts from Patients with Alzheimer's Disease Induce Tau Aggregates in a Cellular Model of Tau Propagation.J Alzheimers Dis Rep. 2021 Apr 6;5(1):263-274. doi: 10.3233/ADR-210298. J Alzheimers Dis Rep. 2021. PMID: 34113783 Free PMC article.
-
Application of single-molecule analysis to singularity phenomenon of cells.Biophys Physicobiol. 2024 May 8;21(Supplemental):e211018. doi: 10.2142/biophysico.bppb-v21.s018. eCollection 2024. Biophys Physicobiol. 2024. PMID: 39175861 Free PMC article.
-
HS3ST2 expression induces the cell autonomous aggregation of tau.Sci Rep. 2022 Jun 27;12(1):10850. doi: 10.1038/s41598-022-13486-6. Sci Rep. 2022. PMID: 35760982 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

