Cyclic Peptides that Govern Signal Transduction Pathways: From Prokaryotes to Multi-Cellular Organisms
- PMID: 29773060
- PMCID: PMC6050129
- DOI: 10.2174/1568026618666180518090705
Cyclic Peptides that Govern Signal Transduction Pathways: From Prokaryotes to Multi-Cellular Organisms
Abstract
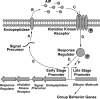
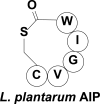
Cyclic peptide scaffolds are key components of signal transduction pathways in both prokaryotic and eukaryotic organisms since they act as chemical messengers that activate or inhibit specific cognate receptors. In prokaryotic organisms these peptides are utilized in non-essential pathways, such as quorum sensing, that are responsible for virulence and pathogenicity. In the more evolved eukaryotic systems, cyclic peptide hormones play a key role in the regulation of the overall function of multicellular organisms, mainly through the endocrine system. This review will highlight several prokaryote and eukaryote systems that use cyclic peptides as their primary signals and the potential associated with utilizing these scaffolds for the discovery of novel therapeutics for a wide range of diseases and illnesses.
Keywords: Cyclic peptides; autoinducers; eukaryotes; hormones; prokaryotes; quorum sensing; signal transduction..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



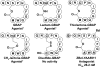
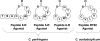

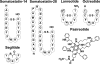

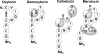
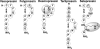
Similar articles
-
Cyclodepsipeptides produced by actinomycetes inhibit cyclic-peptide-mediated quorum sensing in Gram-positive bacteria.FEMS Microbiol Lett. 2015 Jul;362(14):fnv109. doi: 10.1093/femsle/fnv109. Epub 2015 Jul 5. FEMS Microbiol Lett. 2015. PMID: 26149266
-
Quorum sensing: cell-to-cell communication in bacteria.Annu Rev Cell Dev Biol. 2005;21:319-46. doi: 10.1146/annurev.cellbio.21.012704.131001. Annu Rev Cell Dev Biol. 2005. PMID: 16212498 Review.
-
[Mechanism of arsenic compound resistance in prokaryotes and eukaryotes].Postepy Biochem. 1999;45(4):304-12. Postepy Biochem. 1999. PMID: 10786374 Review. Polish. No abstract available.
-
Bacterial social engagements.Trends Cell Biol. 2004 Nov;14(11):648-56. doi: 10.1016/j.tcb.2004.09.012. Trends Cell Biol. 2004. PMID: 15519854 Review.
-
Non-ribosomal Peptide Synthases from Pseudomonas aeruginosa Play a Role in Cyclodipeptide Biosynthesis, Quorum-Sensing Regulation, and Root Development in a Plant Host.Microb Ecol. 2017 Apr;73(3):616-629. doi: 10.1007/s00248-016-0896-4. Epub 2016 Nov 30. Microb Ecol. 2017. PMID: 27900439
Cited by
-
Biological Evaluation of Native Streptococcal Competence Stimulating Peptides Reveal Potential Crosstalk Between Streptococcus mitis and Streptococcus pneumoniae and a New Scaffold for the Development of S. pneumoniae Quorum Sensing Modulators.RSC Chem Biol. 2020 Jun 1;1(2):60-67. doi: 10.1039/D0CB00012D. Epub 2020 May 29. RSC Chem Biol. 2020. PMID: 32905481 Free PMC article.
-
Microbiomics for enhancing electron transfer in an electrochemical system.Front Microbiol. 2022 Jul 29;13:868220. doi: 10.3389/fmicb.2022.868220. eCollection 2022. Front Microbiol. 2022. PMID: 35966693 Free PMC article. Review.
-
Targeting Peptide-Based Quorum Sensing Systems for the Treatment of Gram-Positive Bacterial Infections.Pept Sci (Hoboken). 2023 Mar;115(2):e24298. doi: 10.1002/pep2.24298. Epub 2022 Nov 12. Pept Sci (Hoboken). 2023. PMID: 37397504 Free PMC article.
-
Structural Characterization of Competence-Stimulating Peptide Analogues Reveals Key Features for ComD1 and ComD2 Receptor Binding in Streptococcus pneumoniae.Biochemistry. 2018 Sep 11;57(36):5359-5369. doi: 10.1021/acs.biochem.8b00653. Epub 2018 Aug 28. Biochemistry. 2018. PMID: 30125091 Free PMC article.
-
Characterization of Genomic, Physiological, and Probiotic Features of Lactiplantibacillus plantarum JS21 Strain Isolated from Traditional Fermented Jiangshui.Foods. 2024 Apr 1;13(7):1082. doi: 10.3390/foods13071082. Foods. 2024. PMID: 38611386 Free PMC article.
References
-
- Pawson T, Nash P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000;14(9):1027–1047. - PubMed
-
- Roxin A, Zheng G. Flexible or fixed: a comparative review of linear and cyclic cancer-targeting peptides. Future Med Chem. 2012;4(12):1601–1618. - PubMed
-
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

