PRDX1 and MTH1 cooperate to prevent ROS-mediated inhibition of telomerase
- PMID: 29773556
- PMCID: PMC6004070
- DOI: 10.1101/gad.313460.118
PRDX1 and MTH1 cooperate to prevent ROS-mediated inhibition of telomerase
Abstract
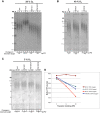
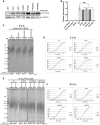
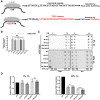
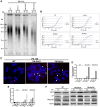
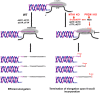
Telomerase counteracts telomere shortening and cellular senescence in germ, stem, and cancer cells by adding repetitive DNA sequences to the ends of chromosomes. Telomeres are susceptible to damage by reactive oxygen species (ROS), but the consequences of oxidation of telomeres on telomere length and the mechanisms that protect from ROS-mediated telomere damage are not well understood. In particular, 8-oxoguanine nucleotides at 3' ends of telomeric substrates inhibit telomerase in vitro, whereas, at internal positions, they suppress G-quadruplex formation and were therefore proposed to promote telomerase activity. Here, we disrupt the peroxiredoxin 1 (PRDX1) and 7,8-dihydro-8-oxoguanine triphosphatase (MTH1) genes in cancer cells and demonstrate that PRDX1 and MTH1 cooperate to prevent accumulation of oxidized guanine in the genome. Concomitant disruption of PRDX1 and MTH1 leads to ROS concentration-dependent continuous shortening of telomeres, which is due to efficient inhibition of telomere extension by telomerase. Our results identify antioxidant systems that are required to protect telomeres from oxidation and are necessary to allow telomere maintenance by telomerase conferring immortality to cancer cells.
Keywords: MTH1; PRDX1; aging; cellular senescence; oxidative stress; telomerase; telomeres.
© 2018 Ahmed and Lingner; Published by Cold Spring Harbor Laboratory Press.
Figures

Comment in
-
Telomerase can't handle the stress.Genes Dev. 2018 May 1;32(9-10):597-599. doi: 10.1101/gad.316042.118. Genes Dev. 2018. PMID: 29802121 Free PMC article.
References
-
- Aeby E, Ahmed W, Redon S, Simanis V, Lingner J. 2016. Peroxiredoxin 1 protects telomeres from oxidative damage and preserves telomeric DNA for extension by telomerase. Cell Rep 17: 3107–3114. - PubMed
-
- Ahmed W, Lingner J. 2018. Impact of oxidative stress on telomere biology. Differentiation 99: 21–27. - PubMed
-
- Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G. 2008. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci 121: 1046–1053. - PubMed
-
- Ancelin K, Brunori M, Bauwens S, Koering CE, Brun C, Ricoul M, Pommier JP, Sabatier L, Gilson E. 2002. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol Cell Biol 22: 3474–3487. - PMC - PubMed
-
- Asai A, Oshima Y, Yamamoto Y, Uochi TA, Kusaka H, Akinaga S, Yamashita Y, Pongracz K, Pruzan R, Wunder E, et al. 2003. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res 63: 3931–3939. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
