Clofarabine, high-dose cytarabine and liposomal daunorubicin in pediatric relapsed/refractory acute myeloid leukemia: a phase IB study
- PMID: 29773602
- PMCID: PMC6119144
- DOI: 10.3324/haematol.2017.187153
Clofarabine, high-dose cytarabine and liposomal daunorubicin in pediatric relapsed/refractory acute myeloid leukemia: a phase IB study
Abstract
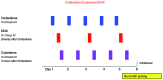
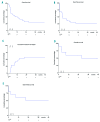
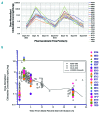
Survival in children with relapsed/refractory acute myeloid leukemia is unsatisfactory. Treatment consists of one course of fludarabine, cytarabine and liposomal daunorubicin, followed by fludarabine and cytarabine and stem-cell transplantation. Study ITCC 020/I-BFM 2009-02 aimed to identify the recommended phase II dose of clofarabine replacing fludarabine in the abovementioned combination regimen (3+3 design). Escalating dose levels of clofarabine (20-40 mg/m2/day × 5 days) and liposomal daunorubicin (40-80 mg/m2/day) were administered with cytarabine (2 g/m2/day × 5 days). Liposomal DNR was given on day 1, 3 and 5 only. The cohort at the recommended phase II dose was expanded to make a preliminary assessment of anti-leukemic activity. Thirty-four children were enrolled: refractory 1st (n=11), early 1st (n=15), ≥2nd relapse (n=8). Dose level 3 (30 mg/m2clofarabine; 60 mg/m2liposomal daunorubicin) appeared to be safe only in patients without subclinical fungal infections. Infectious complications were dose-limiting. The recommended phase II dose was 40 mg/m2 clofarabine with 60 mg/m2 liposomal daunorubicin. Side-effects mainly consisted of infections. The overall response rate was 68% in 31 response evaluable patients, and 80% at the recommended phase II dose (n=10); 22 patients proceeded to stem cell transplantation. The 2-year probability of event-free survival (pEFS) was 26.5±7.6 and probability of survival (pOS) 32.4±8.0%. In the 21 responding patients, the 2-year pEFS was 42.9±10.8 and pOS 47.6±10.9%. Clofarabine exposure in plasma was not significantly different from that in single-agent studies. In conclusion, clofarabine was well tolerated and showed high response rates in relapsed/refractory pediatric acute myeloid leukemia. Patients with (sub) clinical fungal infections should be treated with caution. Clofarabine has been taken forward in the Berlin-Frankfurt-Münster study for newly diagnosed acute myeloid leukemia. The Study ITCC-020 was registered as EUDRA-CT 2009-009457-13; Dutch Trial Registry number 1880.
Copyright© 2018 Ferrata Storti Foundation.
Figures
Similar articles
-
[Liposomal daunorubicine combined with cytarabine in the treatment of relapsed/refractory acute myeloid leukemia in children].Klin Padiatr. 2002 Jul-Aug;214(4):188-94. doi: 10.1055/s-2002-33185. Klin Padiatr. 2002. PMID: 12165900 German.
-
Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group.J Clin Oncol. 2013 Feb 10;31(5):599-607. doi: 10.1200/JCO.2012.43.7384. Epub 2013 Jan 14. J Clin Oncol. 2013. PMID: 23319696 Clinical Trial.
-
Clofarabine, cytarabine, and mitoxantrone in refractory/relapsed acute myeloid leukemia: High response rates and effective bridge to allogeneic hematopoietic stem cell transplantation.Cancer Med. 2020 May;9(10):3371-3382. doi: 10.1002/cam4.2865. Epub 2020 Mar 18. Cancer Med. 2020. PMID: 32187883 Free PMC article. Clinical Trial.
-
The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia.Leuk Lymphoma. 2005 Jun;46(6):795-802. doi: 10.1080/10428190500052438. Leuk Lymphoma. 2005. PMID: 16019523 Review.
-
CPX-351 daunorubicin-cytarabine liposome: a novel formulation to treat patients with newly diagnosed secondary acute myeloid leukemia.Minerva Med. 2020 Oct;111(5):455-466. doi: 10.23736/S0026-4806.20.07017-2. Epub 2020 Sep 21. Minerva Med. 2020. PMID: 32955826 Review.
Cited by
-
Relapsed pediatric acute myeloid leukaemia: state-of-the-art in 2023.Haematologica. 2023 Sep 1;108(9):2275-2288. doi: 10.3324/haematol.2022.281106. Haematologica. 2023. PMID: 36861399 Free PMC article. Review.
-
Clinical outcomes of second relapsed and refractory first relapsed paediatric AML: A retrospective study within the NOPHO-DB SHIP consortium.Br J Haematol. 2022 Jun;197(6):755-765. doi: 10.1111/bjh.18039. Epub 2022 Feb 4. Br J Haematol. 2022. PMID: 35118649 Free PMC article.
-
Re-induction with modified CLAG regimen in relapsed or refractory acute myeloid leukemia in children bridging to allogeneic hematopoietic stem cell transplantation.World J Pediatr. 2020 Apr;16(2):152-158. doi: 10.1007/s12519-019-00321-8. Epub 2019 Nov 20. World J Pediatr. 2020. PMID: 31748985
-
Characteristics of anti-CLL1 based CAR-T therapy for children with relapsed or refractory acute myeloid leukemia: the multi-center efficacy and safety interim analysis.Leukemia. 2022 Nov;36(11):2596-2604. doi: 10.1038/s41375-022-01703-0. Epub 2022 Sep 23. Leukemia. 2022. PMID: 36151140
-
The PedAL/EuPAL Project: A Global Initiative to Address the Unmet Medical Needs of Pediatric Patients with Relapsed or Refractory Acute Myeloid Leukemia.Cancers (Basel). 2023 Dec 22;16(1):78. doi: 10.3390/cancers16010078. Cancers (Basel). 2023. PMID: 38201506 Free PMC article.
References
-
- Gorman MF, Ji L, Ko RH, et al. Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): a Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 2010;55(3):421–429. - PubMed
-
- Kaspers GJ, Zimmermann M, Reinhardt D, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31(5):599–607. - PubMed
-
- Milligan DW, Wheatley K, Littlewood T, Craig JI, Burnett AK, Group NHOCS. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood. 2006;107(12):4614–4622. - PubMed
-
- Ehlers S, Herbst C, Zimmermann M, et al. Granulocyte colony-stimulating factor (G-CSF) treatment of childhood acute myeloid leukemias that overexpress the differentiation-defective G-CSF receptor isoform IV is associated with a higher incidence of relapse. J Clin Oncol. 2010;28(15):2591–2597. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

