Genetic code expansion and live cell imaging reveal that Thr-308 phosphorylation is irreplaceable and sufficient for Akt1 activity
- PMID: 29773654
- PMCID: PMC6036199
- DOI: 10.1074/jbc.RA118.002357
Genetic code expansion and live cell imaging reveal that Thr-308 phosphorylation is irreplaceable and sufficient for Akt1 activity
Abstract
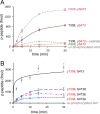
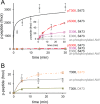
The proto-oncogene Akt/protein kinase B (PKB) is a pivotal signal transducer for growth and survival. Growth factor stimulation leads to Akt phosphorylation at two regulatory sites (Thr-308 and Ser-473), acutely activating Akt signaling. Delineating the exact role of each regulatory site is, however, technically challenging and has remained elusive. Here, we used genetic code expansion to produce site-specifically phosphorylated Akt1 to dissect the contribution of each regulatory site to Akt1 activity. We achieved recombinant production of full-length Akt1 containing site-specific pThr and pSer residues for the first time. Our analysis of Akt1 site-specifically phosphorylated at either or both sites revealed that phosphorylation at both sites increases the apparent catalytic rate 1500-fold relative to unphosphorylated Akt1, an increase attributable primarily to phosphorylation at Thr-308. Live imaging of COS-7 cells confirmed that phosphorylation of Thr-308, but not Ser-473, is required for cellular activation of Akt. We found in vitro and in the cell that pThr-308 function cannot be mimicked with acidic residues, nor could unphosphorylated Thr-308 be mimicked by an Ala mutation. An Akt1 variant with pSer-308 achieved only partial enzymatic and cellular signaling activity, revealing a critical interaction between the γ-methyl group of pThr-308 and Cys-310 in the Akt1 active site. Thus, pThr-308 is necessary and sufficient to stimulate Akt signaling in cells, and the common use of phosphomimetics is not appropriate for studying the biology of Akt signaling. Our data also indicate that pThr-308 should be regarded as the primary diagnostic marker of Akt activity.
Keywords: Akt/protein kinase B (PKB); activation loop; aminoacyl tRNA synthetase; cell biology; cell signaling; enzyme; genetic code expansion; hydrophobic motif; phosphomimetics; phosphoseryl-tRNA synthetase (SepRS); protein phosphorylation; tRNASep; transfer RNA (tRNA).
© 2018 Balasuriya et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

