miR126-5p Downregulation Facilitates Axon Degeneration and NMJ Disruption via a Non-Cell-Autonomous Mechanism in ALS
- PMID: 29773756
- PMCID: PMC6001038
- DOI: 10.1523/JNEUROSCI.3037-17.2018
miR126-5p Downregulation Facilitates Axon Degeneration and NMJ Disruption via a Non-Cell-Autonomous Mechanism in ALS
Abstract
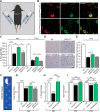
Axon degeneration and disruption of neuromuscular junctions (NMJs) are key events in amyotrophic lateral sclerosis (ALS) pathology. Although the disease's etiology is not fully understood, it is thought to involve a non-cell-autonomous mechanism and alterations in RNA metabolism. Here, we identified reduced levels of miR126-5p in presymptomatic ALS male mice models, and an increase in its targets: axon destabilizing Type 3 Semaphorins and their coreceptor Neuropilins. Using compartmentalized in vitro cocultures, we demonstrated that myocytes expressing diverse ALS-causing mutations promote axon degeneration and NMJ dysfunction, which were inhibited by applying Neuropilin1 blocking antibody. Finally, overexpressing miR126-5p is sufficient to transiently rescue axon degeneration and NMJ disruption both in vitro and in vivo Thus, we demonstrate a novel mechanism underlying ALS pathology, in which alterations in miR126-5p facilitate a non-cell-autonomous mechanism of motor neuron degeneration in ALS.SIGNIFICANCE STATEMENT Despite some progress, currently no effective treatment is available for amyotrophic lateral sclerosis (ALS). We suggest a novel regulatory role for miR126-5p in ALS and demonstrate, for the first time, a mechanism by which alterations in miR126-5p contribute to axon degeneration and NMJ disruption observed in ALS. We show that miR126-5p is altered in ALS models and that it can modulate Sema3 and NRP protein expression. Furthermore, NRP1 elevations in motor neurons and muscle secretion of Sema3A contribute to axon degeneration and NMJ disruption in ALS. Finally, overexpressing miR126-5p is sufficient to transiently rescue NMJ disruption and axon degeneration both in vitro and in vivo.
Keywords: ALS; NMJ; Sema3A; axon degeneration; miRNA; microfluidic chambers.
Copyright © 2018 Maimon et al.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
