The Role of Brincidofovir in Preparation for a Potential Smallpox Outbreak
- PMID: 29773767
- PMCID: PMC5707527
- DOI: 10.3390/v9110320
The Role of Brincidofovir in Preparation for a Potential Smallpox Outbreak
Abstract
Smallpox (variola) virus is considered a Category A bioterrorism agent due to its ability to spread rapidly and the high morbidity and mortality rates associated with infection. Current recommendations recognize the importance of oral antivirals and call for having at least two smallpox antivirals with different mechanisms of action available in the event of a smallpox outbreak. Multiple antivirals are recommended due in large part to the propensity of viruses to become resistant to antiviral therapy, especially monotherapy. Advances in synthetic biology heighten concerns that a bioterror attack with variola would utilize engineered resistance to antivirals and potentially vaccines. Brincidofovir, an oral antiviral in late stage development, has proven effective against orthopoxviruses in vitro and in vivo, has a different mechanism of action from tecovirimat (the only oral smallpox antiviral currently in the US Strategic National Stockpile), and has a resistance profile that reduces concerns in the scenario of a bioterror attack using genetically engineered smallpox. Given the devastating potential of smallpox as a bioweapon, preparation of a multi-pronged defense that accounts for the most obvious bioengineering possibilities is strategically imperative.
Keywords: CMX001; antiviral; bioterrorism; bioweapon; brincidofovir; smallpox; variola virus.
Conflict of interest statement
Scott A. Foster and Randall Lanier are employees of Chimerix, the developer of brincidofovir. Scott Parker has provided consulting services to Chimerix. The authors declare no other conflict of interest.
Figures
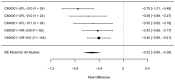
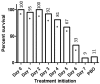
References
-
- Gellman B. 4 nations thought to possess smallpox: Iraq, N. Korea named, two officials say. Washington Post. Nov 5, 2001. p. A1.
-
- Alibek K., Handelman S. Biohazard: The Chilling True Story of the Largest Biological Weapons Program in the World. Random House; New York, NY, USA: 1999. pp. 107–122.
-
- Arvin A.M., Patel D.M. Live Variola Virus: Considerations for Continuing Research. The National Academies Press; Washington, DC, USA: 2009. p. 133.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

