Ultracold Rydberg molecules
- PMID: 29773795
- PMCID: PMC5958105
- DOI: 10.1038/s41467-018-04135-6
Ultracold Rydberg molecules
Abstract
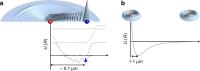
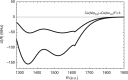
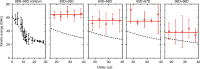
Ultracold molecules formed from association of a single Rydberg atom with surrounding atoms or molecules and those from double Rydberg excitations are discussed in this review. Ultralong-range Rydberg molecules possess a novel molecular bond resulting from scattering of the Rydberg electron from the perturber atoms or molecules. The strong interactions between Rydberg atoms in ultracold gases may lead to formation of macroscopic Rydberg macrodimers. The exquisite control over the properties of the Rydberg electron means that interesting and unusual few-body and quantum many-body features can be realized in such systems.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Balmer JJ. Notiz über die spektrallinien des wasserstoffs. Ann. Physik Chem. N. F. 1885;25:80–87.
-
- Rydberg JH. Den. K. Sven. Vetensk. Handl. 1889;23:11.
-
- Gallagher, T. F. Rydberg Atoms (Cambridge University Press, 2005).
-
- Amaldi E, Segre E. Effetto della pressione sui termini elevati degli alcalini. Il Nuovo Cim. (1924–1942) 1934;11:145–156.
-
- Fermi E. Sopra lo spostamento per pressione delle righe elevate delle serie spettrali. Il Nuovo Cim. (1924–1942) 1934;11:157–166.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

