A novel small molecule chaperone of rod opsin and its potential therapy for retinal degeneration
- PMID: 29773803
- PMCID: PMC5958115
- DOI: 10.1038/s41467-018-04261-1
A novel small molecule chaperone of rod opsin and its potential therapy for retinal degeneration
Abstract
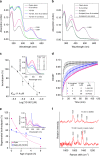
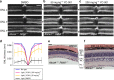
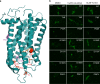
Rhodopsin homeostasis is tightly coupled to rod photoreceptor cell survival and vision. Mutations resulting in the misfolding of rhodopsin can lead to autosomal dominant retinitis pigmentosa (adRP), a progressive retinal degeneration that currently is untreatable. Using a cell-based high-throughput screen (HTS) to identify small molecules that can stabilize the P23H-opsin mutant, which causes most cases of adRP, we identified a novel pharmacological chaperone of rod photoreceptor opsin, YC-001. As a non-retinoid molecule, YC-001 demonstrates micromolar potency and efficacy greater than 9-cis-retinal with lower cytotoxicity. YC-001 binds to bovine rod opsin with an EC50 similar to 9-cis-retinal. The chaperone activity of YC-001 is evidenced by its ability to rescue the transport of multiple rod opsin mutants in mammalian cells. YC-001 is also an inverse agonist that non-competitively antagonizes rod opsin signaling. Significantly, a single dose of YC-001 protects Abca4 -/- Rdh8 -/- mice from bright light-induced retinal degeneration, suggesting its broad therapeutic potential.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Thompson DA, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2000;41:4293–4299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

