DNAJC17 is localized in nuclear speckles and interacts with splicing machinery components
- PMID: 29773831
- PMCID: PMC5958099
- DOI: 10.1038/s41598-018-26093-1
DNAJC17 is localized in nuclear speckles and interacts with splicing machinery components
Abstract
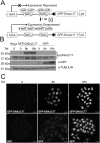
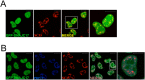
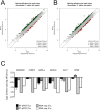
DNAJC17 is a heat shock protein (HSP40) family member, identified in mouse as susceptibility gene for congenital hypothyroidism. DNAJC17 knockout mouse embryos die prior to implantation. In humans, germline homozygous mutations in DNAJC17 have been found in syndromic retinal dystrophy patients, while heterozygous mutations represent candidate pathogenic events for myeloproliferative disorders. Despite widespread expression and involvement in human diseases, DNAJC17 function is still poorly understood. Herein, we have investigated its function through high-throughput transcriptomic and proteomic approaches. DNAJC17-depleted cells transcriptome highlighted genes involved in general functional categories, mainly related to gene expression. Conversely, DNAJC17 interactome can be classified in very specific functional networks, with the most enriched one including proteins involved in splicing. Furthermore, several splicing-related interactors, were independently validated by co-immunoprecipitation and in vivo co-localization. Accordingly, co-localization of DNAJC17 with SC35, a marker of nuclear speckles, further supported its interaction with spliceosomal components. Lastly, DNAJC17 up-regulation enhanced splicing efficiency of minigene reporter in live cells, while its knockdown induced perturbations of splicing efficiency at whole genome level, as demonstrated by specific analysis of RNAseq data. In conclusion, our study strongly suggests a role of DNAJC17 in splicing-related processes and provides support to its recognized essential function in early development.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

