The Cytotoxic Effects of Betulin-Conjugated Gold Nanoparticles as Stable Formulations in Normal and Melanoma Cells
- PMID: 29773989
- PMCID: PMC5943567
- DOI: 10.3389/fphar.2018.00429
The Cytotoxic Effects of Betulin-Conjugated Gold Nanoparticles as Stable Formulations in Normal and Melanoma Cells
Abstract
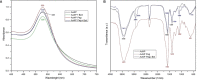
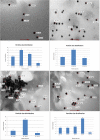
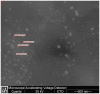
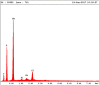
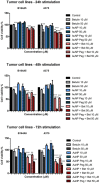
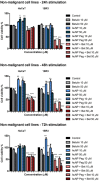
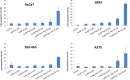
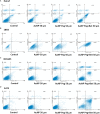
Gold nanoparticles are currently investigated as theranostics tools in cancer therapy due to their proper biocompatibility and increased efficacy related to the ease to customize the surface properties and to conjugate other molecules. Betulin, [lup-20(29)-ene-3β, 28-diol], is a pentacyclic triterpene that has raised scientific interest due to its antiproliferative effect on several cancer types. Herein we described the synthesis of surface modified betulin-conjugated gold nanoparticles using a slightly modified Turkevich method. Transmission electron microscopy (TEM) imaging, dynamic light scattering (DLS), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) were used for the characterization of obtained gold nanoparticles. Cytotoxic activity and apoptosis assessment were carried out using the MTT and Annexin V/PI apoptosis assays. The in vitro results showed that betulin coated gold nanoparticles presented a dose-dependent cytotoxic effect and induced apoptosis in all tested cell lines.
Keywords: apoptosis; betulin; gold nanoparticles; melanoma; skin.
Figures
References
-
- Agunloye E., Gavriilidis A., Mazzei L. (2017). A mathematical investigation of the Turkevich organizer theory in the citrate method for the synthesis of gold nanoparticles. Chem. Eng. Sci. 173 275–286. 10.1016/j.ces.2017.07.032 - DOI
-
- Alkilany A. M., Bani Yaseen A. I., Kailani M. H. (2015). Synthesis of monodispersed gold nanoparticles with exceptional colloidal stability with grafted polyethylene glycol-g-polyvinyl alcohol. J. Nanomater. 16:51 10.1155/2015/712359 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

