Tumoral cavitation in colorectal cancer patients with unresectable lung metastasis treated with bevacizumab and chemotherapy
- PMID: 29774414
- PMCID: PMC6002465
- DOI: 10.1007/s00432-018-2656-y
Tumoral cavitation in colorectal cancer patients with unresectable lung metastasis treated with bevacizumab and chemotherapy
Abstract
Purpose: Efficacy and the frequency of tumor cavitation have not been investigated in patients with metastatic colorectal cancer (mCRC) and lung metastases (LMs) treated with chemotherapy in combination with bevacizumab. This study was aimed to evaluate the efficacy and safety of bevacizumab for unresectable mCRC with LM and to determine the frequency of tumor cavitation, and its correlation with clinical outcomes in patients receiving bevacizumab plus chemotherapy.
Methods: Patients with mCRC and LMs treated with bevacizumab as first- or second-line therapy at West China Hospital, Sichuan University Cancer Center from September 2010 to November 2016 were included in this retrospective study. Data on clinicopathological characteristic were collected and overall survival (OS), progression-free survival (PFS), objective response rate (ORR) and disease control rate (DCR) were determined.
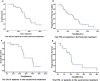
Results: Among 60 patients included in the study, response rate (RR), stable disease (SD), and DCR were 43.6% (17/39), 51.3% (20/39) and 94.9% (37/39) in patients receiving bevacizumab as first-line treatment. Median OS and PFS of the first-line treatment group were 32.4 and 15.5 months, respectively. Among 60, 12 patients (20%) developed cavitation after bevacizumab therapy initiation. Median OS was longer in patients with cavitation than those without cavitation (42.1 vs 30.8 months; p = 0.042) in the first-line treatment group.
Conclusion: Bevacizumab in combination with chemotherapy exhibited promising efficacy in mCRC patients LMs. Moreover, our findings reveal that OS might be affected by new tumor cavitation during antiangiogenic agent treatment.
Keywords: Antiangiogenic agent; Bevacizumab; Cavitation; Colorectal cancer; Lung metastasis.
Conflict of interest statement
Author Hongfeng Gou declares that she has no conflict of interest. Author Yu Peng declares that she has no conflict of interest. Author Ye Chen declares that she has no conflict of interest. Author Xi Zhang declares that he has no conflict of interest. Author Yu Yang declares that she has no conflict of interest. Author Dan Cao declares that she has no conflict of interest. Author Feng Bi declares that he has no conflict of interest. Author Zhiping Li declares that he has no conflict of interest.
Figures


References
-
- Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard J-Y, Lathia C, SchwartzB et al (2006) Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24:4293–300 - PubMed
-
- Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Charnsangavej C (2007) We should desist using RECIST, at least in GIST. J Clin Oncol 25:1760–1764 - PubMed
-
- Chen WQ, Zheng RS, Zhang SW, Zeng HW, Zou XN, HeJ (2017) Analysis of cancer incidence and mortality in China, 2013. Zhong guo Zhong Liu 23:1–10 - PubMed
-
- Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR et al (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25:1753–1759 - PubMed
-
- Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G et al (2009) Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol 27:404–410 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

