Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain
- PMID: 29774535
- PMCID: PMC6265560
- DOI: 10.1113/JP275376
Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain
Abstract
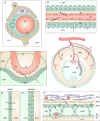
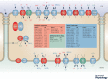
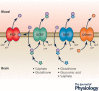
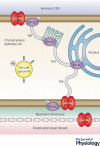
Properties of the local internal environment of the adult brain are tightly controlled providing a stable milieu essential for its normal function. The mechanisms involved in this complex control are structural, molecular and physiological (influx and efflux transporters) frequently referred to as the 'blood-brain barrier'. These mechanisms include regulation of ion levels in brain interstitial fluid essential for normal neuronal function, supply of nutrients, removal of metabolic products, and prevention of entry or elimination of toxic agents. A key feature is cerebrospinal fluid secretion and turnover. This is much less during development, allowing greater accumulation of permeating molecules. The overall effect of these mechanisms is to tightly control the exchange of molecules into and out of the brain. This review presents experimental evidence currently available on the status of these mechanisms in developing brain. It has been frequently stated for over nearly a century that the blood-brain barrier is not present or at least is functionally deficient in the embryo, fetus and newborn. We suggest the alternative hypothesis that the barrier mechanisms in developing brain are likely to be appropriately matched to each stage of its development. The contributions of different barrier mechanisms, such as changes in constituents of cerebrospinal fluid in relation to specific features of brain development, for example neurogenesis, are only beginning to be studied. The evidence on this previously neglected aspect of brain barrier function is outlined. We also suggest future directions this field could follow with special emphasis on potential applications in a clinical setting.
Keywords: amino acids; blood vessels; blood-brain barrier; cerebrospinal fluid; choroid plexus; electrolytes; electron microscope; embryo; endothelium; epithelium; gene transcripts; immunohistochemistry; ion gradients; meninges; protein; tight junctions; transport.
© 2018 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures







References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR & Begley DJ (2010). Structure and function of the blood‐brain barrier. Neurobiol Dis 37, 13–25. - PubMed
-
- Adinolfi M (1985). The development of the human blood‐CSF‐brain barrier. Dev Med Child Neurol 27, 532–537. - PubMed
-
- Adinolfi M & Haddad SA (1977). Levels of plasma proteins in human and rat fetal CSF and the development of the blood‐CSF barrier. Neuropadiatrie 8, 345–353. - PubMed
-
- Akiyama T, Kobayashi K, Higashikage A, Sato J & Yoshinaga H (2014). CSF/plasma ratios of amino acids: reference data and transports in children. Brain Dev 36, 3–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

