Diabetes-induced microvascular complications at the level of the spinal cord: a contributing factor in diabetic neuropathic pain
- PMID: 29774557
- PMCID: PMC6092307
- DOI: 10.1113/JP275067
Diabetes-induced microvascular complications at the level of the spinal cord: a contributing factor in diabetic neuropathic pain
Abstract
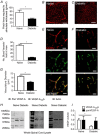
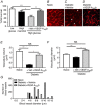
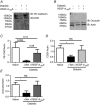
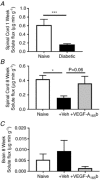
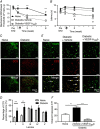
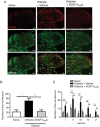
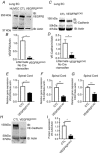
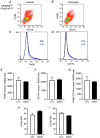
Key points: Diabetes is thought to induce neuropathic pain through activation of dorsal horn sensory neurons in the spinal cord. Here we explore the impact of hyperglycaemia on the blood supply supporting the spinal cord and chronic pain development. In streptozotocin-induced diabetic rats, neuropathic pain is accompanied by a decline in microvascular integrity in the dorsal horn. Hyperglycaemia-induced degeneration of the endothelium in the dorsal horn was associated with a loss in vascular endothelial growth factor (VEGF)-A165 b expression. VEGF-A165 b treatment prevented diabetic neuropathic pain and degeneration of the endothelium in the spinal cord. Using an endothelial-specific VEGFR2 knockout transgenic mouse model, the loss of endothelial VEGFR2 signalling led to a decline in vascular integrity in the dorsal horn and the development of hyperalgesia in VEGFR2 knockout mice. This highlights that vascular degeneration in the spinal cord could be a previously unidentified factor in the development of diabetic neuropathic pain.
Abstract: Abnormalities of neurovascular interactions within the CNS of diabetic patients is associated with the onset of many neurological disease states. However, to date, the link between the neurovascular network within the spinal cord and regulation of nociception has not been investigated despite neuropathic pain being common in diabetes. We hypothesised that hyperglycaemia-induced endothelial degeneration in the spinal cord, due to suppression of vascular endothelial growth factor (VEGF)-A/VEGFR2 signalling, induces diabetic neuropathic pain. Nociceptive pain behaviour was investigated in a chemically induced model of type 1 diabetes (streptozotocin induced, insulin supplemented; either vehicle or VEGF-A165 b treated) and an inducible endothelial knockdown of VEGFR2 (tamoxifen induced). Diabetic animals developed mechanical allodynia and heat hyperalgesia. This was associated with a reduction in the number of blood vessels and reduction in Evans blue extravasation in the lumbar spinal cord of diabetic animals versus age-matched controls. Endothelial markers occludin, CD31 and VE-cadherin were downregulated in the spinal cord of the diabetic group versus controls, and there was a concurrent reduction of VEGF-A165 b expression. In diabetic animals, VEGF-A165 b treatment (biweekly i.p., 20 ng g-1 ) restored normal Evans blue extravasation and prevented vascular degeneration, diabetes-induced central neuron activation and neuropathic pain. Inducible knockdown of VEGFR2 (tamoxifen treated Tie2CreERT2 -vegfr2flfl mice) led to a reduction in blood vessel network volume in the lumbar spinal cord and development of heat hyperalgesia. These findings indicate that hyperglycaemia leads to a reduction in the VEGF-A/VEGFR2 signalling cascade, resulting in endothelial dysfunction in the spinal cord, which could be an undiscovered contributing factor to diabetic neuropathic pain.
Keywords: diabetes; endothelial; pain.
© 2018 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures

Comment in
-
VEGF-A165 b to the rescue: vascular integrity and pain sensitization.J Physiol. 2018 Nov;596(21):5077-5078. doi: 10.1113/JP276902. Epub 2018 Sep 19. J Physiol. 2018. PMID: 30156272 Free PMC article. No abstract available.
References
-
- Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, Takeda A, Baffi JZ, Yamada K, Kaneko H, Green MG, Chappell J, Wilting J, Weich HA, Yamagami S, Amano S, Mizuki N, Alexander JS, Peterson ML, Brekken RA, Hirashima M, Capoor S, Usui T, Ambati BK & Ambati J (2009). Alternatively spliced vascular endothelial growth factor receptor‐2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med 15, 1023–1030. - PMC - PubMed
-
- Bates D, Cui T, Doughty J, Winkler M, Sugiono M, Shields J, Peat D, Gillatt D & Harper S (2002). VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down‐regulated in renal cell carcinoma. Cancer Res 62, 4123–4131. - PubMed
-
- Beazley‐Long N, Ashby WR, Moss CE, Bestall SM, Almahasneh F, AeM Durrant, Benest AV, Blackley Z, Ballmer‐Hofer K, Hirashima M, Hulse RP, Bates DO & Donaldson LF (2018). VEGFR2 promotes central endothelial activation and the spread of pain in inflammatory arthritis. Brain Behav Immun, 10.1016/j.bbi.2018.03.012 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

