Loss of sirtuin 1 and mitofusin 2 contributes to enhanced ischemia/reperfusion injury in aged livers
- PMID: 29774638
- PMCID: PMC6052398
- DOI: 10.1111/acel.12761
Loss of sirtuin 1 and mitofusin 2 contributes to enhanced ischemia/reperfusion injury in aged livers
Abstract
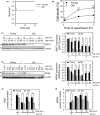
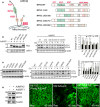
Ischemia/reperfusion (I/R) injury is a causative factor contributing to morbidity and mortality during liver resection and transplantation. Livers from elderly patients have a poorer recovery from these surgeries, indicating reduced reparative capacity with aging. Mechanisms underlying this age-mediated hypersensitivity to I/R injury remain poorly understood. Here, we investigated how sirtuin 1 (SIRT1) and mitofusin 2 (MFN2) are affected by I/R in aged livers. Young (3 months) and old (23-26 months) male C57/BL6 mice were subjected to hepatic I/R in vivo. Primary hepatocytes isolated from each age group were also exposed to simulated in vitro I/R. Biochemical, genetic, and imaging analyses were performed to assess cell death, autophagy flux, mitophagy, and mitochondrial function. Compared to young mice, old livers showed accelerated liver injury following mild I/R. Reperfusion of old hepatocytes also showed necrosis, accompanied with defective autophagy, onset of the mitochondrial permeability transition, and mitochondrial dysfunction. Biochemical analysis indicated a near-complete loss of both SIRT1 and MFN2 after I/R in old hepatocytes, which did not occur in young cells. Overexpression of either SIRT1 or MFN2 alone in old hepatocytes failed to mitigate I/R injury, while co-overexpression of both proteins promoted autophagy and prevented mitochondrial dysfunction and cell death after reperfusion. Genetic approaches with deletion and point mutants revealed that SIRT1 deacetylated K655 and K662 residues in the C-terminus of MFN2, leading to autophagy activation. The SIRT1-MFN2 axis is pivotal during I/R recovery and may be a novel therapeutic target to reduce I/R injury in aged livers.
Keywords: aging; autophagy; hepatocytes; liver; mitochondria; mitophagy.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures




References
-
- Atkins, K. M. , Thomas, L. L. , Barroso‐González, J. , Thomas, L. , Auclair, S. , Yin, J. , … Thomas, G. (2014). The multifunctional sorting protein PACS‐2 regulates SIRT1‐mediated deacetylation of p53 to modulate p21‐dependent cell‐cycle arrest. Cell Reports, 8, 1545–1557. 10.1016/j.celrep.2014.07.049 - DOI - PMC - PubMed
-
- Bach, D. , Pich, S. , Soriano, F. X. , Vega, N. , Baumgartner, B. , Oriola, J. , … Zorzano, A. (2003). Mitofusin‐2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. Journal of Biological Chemistry, 278, 17190–17197. 10.1074/jbc.M212754200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

