CTC1-STN1 coordinates G- and C-strand synthesis to regulate telomere length
- PMID: 29774655
- PMCID: PMC6052479
- DOI: 10.1111/acel.12783
CTC1-STN1 coordinates G- and C-strand synthesis to regulate telomere length
Abstract
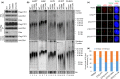
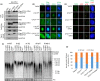
Coats plus (CP) is a rare autosomal recessive disorder caused by mutations in CTC1, a component of the CST (CTC1, STN1, and TEN1) complex important for telomere length maintenance. The molecular basis of how CP mutations impact upon telomere length remains unclear. The CP CTC1L1142H mutation has been previously shown to disrupt telomere maintenance. In this study, we used CRISPR/Cas9 to engineer this mutation into both alleles of HCT116 and RPE cells to demonstrate that CTC1:STN1 interaction is required to repress telomerase activity. CTC1L1142H interacts poorly with STN1, leading to telomerase-mediated telomere elongation. Impaired interaction between CTC1L1142H :STN1 and DNA Pol-α results in increased telomerase recruitment to telomeres and further telomere elongation, revealing that C:S binding to DNA Pol-α is required to fully repress telomerase activity. CP CTC1 mutants that fail to interact with DNA Pol-α resulted in loss of C-strand maintenance and catastrophic telomere shortening. Our findings place the CST complex as an important regulator of both G-strand extensions by telomerase and C-strand synthesis by DNA Pol-α.
Keywords: DNA repair; stem cell aging; telomerase; telomere.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures
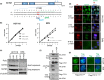
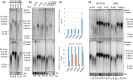
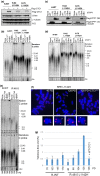
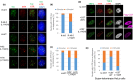

References
-
- Bisht, K. , Smith, E. M. , Tesmer, V. M. , & Nandakumar, J. (2016). Structural and functional consequences of a disease mutation in the telomere protein TPP1. Proceedings of the National Academy of Sciences of the United States of America, 113, 13021–13026. 10.1073/pnas.1605685113 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AG000114/AG/NIA NIH HHS/United States
- R01 CA202816/CA/NCI NIH HHS/United States
- R00 CA167644/CA/NCI NIH HHS/United States
- R21 CA200506/CA/NCI NIH HHS/United States
- T32 GM007205/GM/NIGMS NIH HHS/United States
- R01 AG050509/AG/NIA NIH HHS/United States
- RSG-17-037-01-DMC/American Cancer Society Research Scholar/International
- R01 GM120094/GM/NIGMS NIH HHS/United States
- R21 CA182280/CA/NCI NIH HHS/United States
- R01 GM088351/GM/NIGMS NIH HHS/United States
- R01 CA129037/CA/NCI NIH HHS/United States
- R01 CA190492/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

