Occupation-Associated Fatal Limbic Encephalitis Caused by Variegated Squirrel Bornavirus 1, Germany, 2013
- PMID: 29774846
- PMCID: PMC6004865
- DOI: 10.3201/eid2406.172027
Occupation-Associated Fatal Limbic Encephalitis Caused by Variegated Squirrel Bornavirus 1, Germany, 2013
Abstract
Limbic encephalitis is commonly regarded as an autoimmune-mediated disease. However, after the recent detection of zoonotic variegated squirrel bornavirus 1 in a Prevost's squirrel (Callosciurus prevostii) in a zoo in northern Germany, we retrospectively investigated a fatal case in an autoantibody-seronegative animal caretaker who had worked at that zoo. The virus had been discovered in 2015 as the cause of a cluster of cases of fatal encephalitis among breeders of variegated squirrels (Sciurus variegatoides) in eastern Germany. Molecular assays and immunohistochemistry detected a limbic distribution of the virus in brain tissue of the animal caretaker. Phylogenetic analyses demonstrated a spillover infection from the Prevost's squirrel. Antibodies against bornaviruses were detected in the patient's cerebrospinal fluid by immunofluorescence and newly developed ELISAs and immunoblot. The putative antigenic epitope was identified on the viral nucleoprotein. Other zoo workers were not infected; however, avoidance of direct contact with exotic squirrels and screening of squirrels are recommended.
Keywords: Bornavirus; Germany; VSBV-1; limbic encephalitis; occupational risk; squirrel; transmission; variegated squirrel bornavirus 1; viruses; zoonoses.
Figures
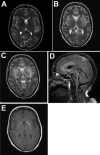
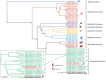


References
-
- Brierley JB, Corsellis JAN, Hierons R, Nevin S. Subacute encephalitis of later adult life mainly affecting the limbic areas. Brain. 1960;83:357–68. 10.1093/brain/83.3.357 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

