The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress
- PMID: 29775595
- PMCID: PMC5967263
- DOI: 10.1016/j.cell.2018.03.037
The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress
Abstract
Chronic social isolation causes severe psychological effects in humans, but their neural bases remain poorly understood. 2 weeks (but not 24 hr) of social isolation stress (SIS) caused multiple behavioral changes in mice and induced brain-wide upregulation of the neuropeptide tachykinin 2 (Tac2)/neurokinin B (NkB). Systemic administration of an Nk3R antagonist prevented virtually all of the behavioral effects of chronic SIS. Conversely, enhancing NkB expression and release phenocopied SIS in group-housed mice, promoting aggression and converting stimulus-locked defensive behaviors to persistent responses. Multiplexed analysis of Tac2/NkB function in multiple brain areas revealed dissociable, region-specific requirements for both the peptide and its receptor in different SIS-induced behavioral changes. Thus, Tac2 coordinates a pleiotropic brain state caused by SIS via a distributed mode of action. These data reveal the profound effects of prolonged social isolation on brain chemistry and function and suggest potential new therapeutic applications for Nk3R antagonists.
Keywords: BNST; DMH; Nk3R; NkB; Tac2; aggression; amygdala; fear; neuropeptides; social isolation; stress.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests. B.E.D. and V.G. are listed as inventors on a patent related to AAV-PHP.B (#US9585971B2).
Figures

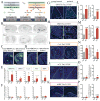



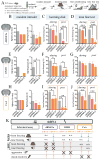

Comment in
-
Social Isolation Co-opts Fear and Aggression Circuits.Cell. 2018 May 17;173(5):1071-1072. doi: 10.1016/j.cell.2018.04.031. Cell. 2018. PMID: 29775590
-
A molecular signature for social isolation identified in the brain.Nature. 2018 Jul;559(7712):38-40. doi: 10.1038/d41586-018-05447-9. Nature. 2018. PMID: 29959409 No abstract available.
References
-
- An D, Chen W, Yu DQ, Wang SW, Yu WZ, Xu H, Wang DM, Zhao D, Sun YP, Wu JC, Tang YY, Yin SM. Effects of social isolation, resocialization and age on cognitive and aggressive behaviors of Kunming mice and BALB/c mice. Animal science journal = Nihon chikusan Gakkaiho. 2017;88:798–806. - PubMed
-
- Anderson DJ. Circuit modules linking internal states and social behaviour in flies and mice. Nature reviews Neuroscience. 2016;17:692–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

