Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3
- PMID: 29776446
- PMCID: PMC5960086
- DOI: 10.1186/s12974-018-1193-6
Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3
Abstract
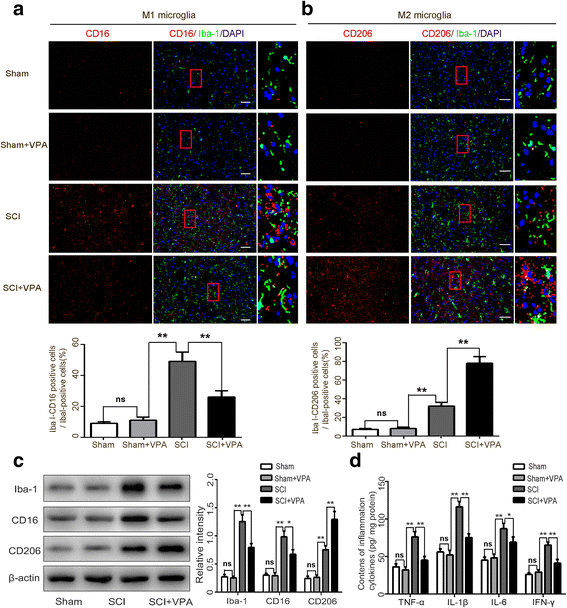
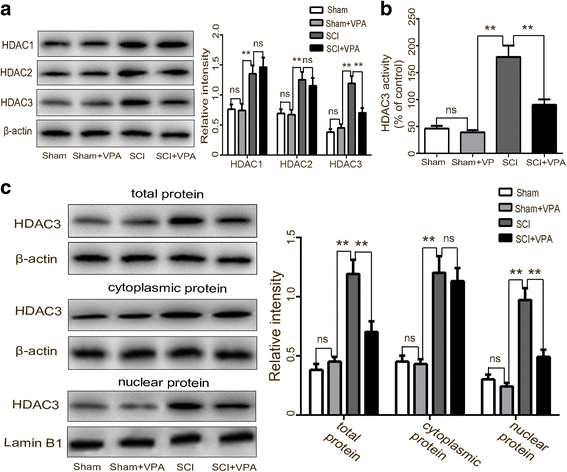
Background: Microglial polarization with M1/M2 phenotype shifts and the subsequent neuroinflammatory responses are vital contributing factors for spinal cord injury (SCI)-induced secondary injury. Nuclear factor-κB (NF-κB) is considered the central transcription factor of inflammatory mediators, which plays a crucial role in microglial activation. Lysine acetylation of STAT1 seems necessary for NF-kB pathway activity, as it is regulated by histone deacetylases (HDACs). There have been no studies that have explained if HDAC inhibition by valproic acid (VPA) affects the NF-κB pathway via acetylation of STAT1 dependent of HDAC activity in the microglia-mediated central inflammation following SCI. We investigated the potential molecular mechanisms that focus on the phenotypic transition of microglia and the STAT1-mediated NF-κB acetylation after a VPA treatment.
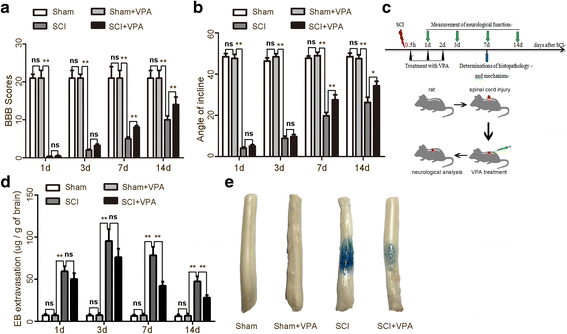
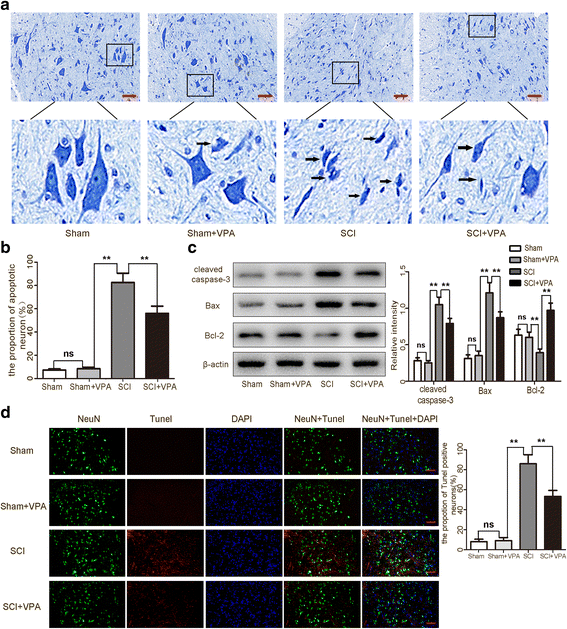
Methods: The Basso-Beattie-Bresnahan locomotion scale, the inclined plane test, the blood-spinal cord barrier, and Nissl staining were employed to determine the neuroprotective effects of VPA treatment after SCI. Assessment of microglia polarization and pro-inflammatory markers, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and interferon (INF)-γ was used to evaluate the neuroinflammatory responses and the anti-inflammatory effects of VPA treatment. Immunofluorescent staining and Western blot analysis were used to detect HDAC3 nuclear translocation, activity, and NF-κB signaling pathway activation to evaluate the effects of VPA treatment. The impact of STAT1 acetylation on NF-kB pathway and the interaction between STAT1 and NF-kB were assessed to evaluate anti-inflammation effects of VPA treatment and also whether these effects were dependent on a STAT1/NF-κB pathway to gain further insight into the mechanisms underlying the development of the neuroinflammatory response after SCI.
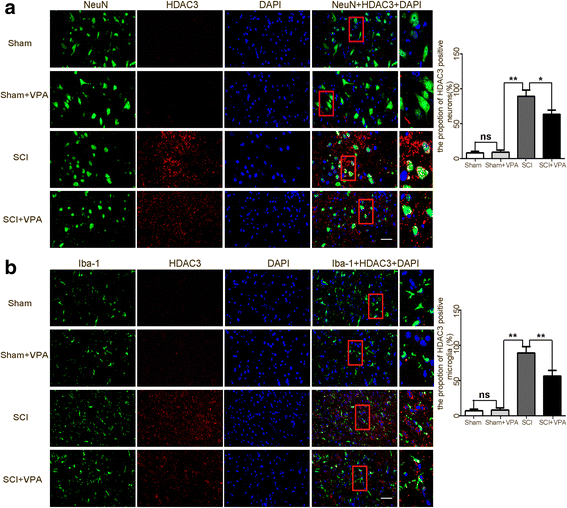
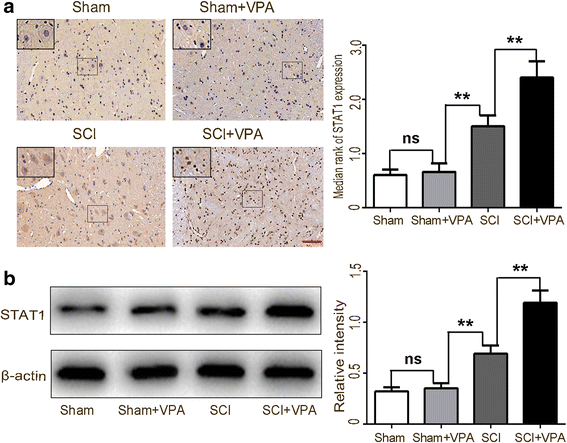
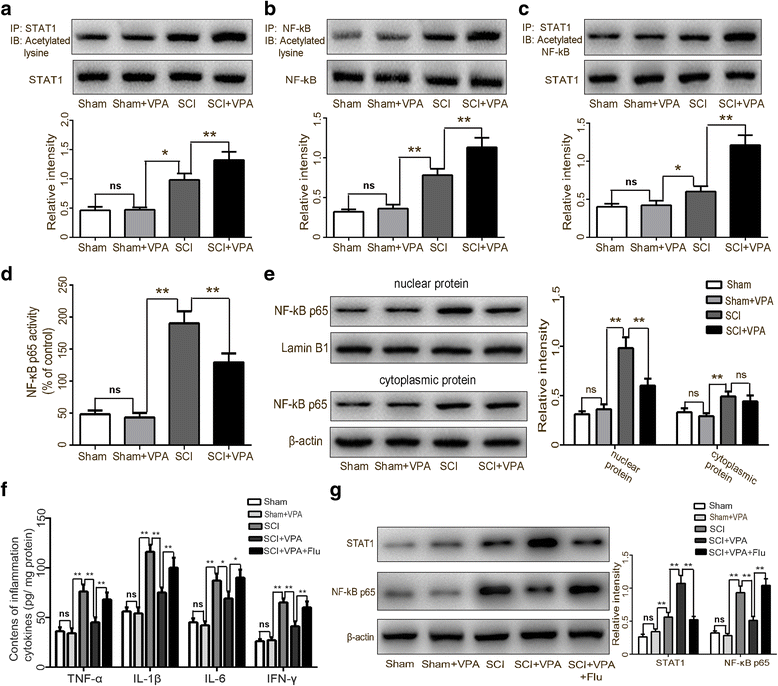
Results: The results showed that the VPA treatment promoted the phenotypic shift of microglia from M1 to M2 phenotype and inhibited microglial activation, thus reducing the SCI-induced inflammatory factors. The VPA treatment upregulation of the acetylation of STAT1/NF-κB pathway was likely caused by the HDAC3 translocation to the nucleus and activity. These results indicated that the treatment with the VPA suppressed the expression and the activity of HDAC3 and enhanced STAT1, as well as NF-κB p65 acetylation following a SCI. The acetylation status of NF-kB p65 and the complex with NF-κB p65 and STAT1 inhibited the NF-kB p65 transcriptional activity and attenuated the microglia-mediated central inflammatory response following SCI.
Conclusions: These results suggested that the VPA treatment attenuated the inflammatory response by modulating microglia polarization through STAT1-mediated acetylation of the NF-κB pathway, dependent of HDAC3 activity. These effects led to neuroprotective effects following SCI.
Keywords: HDAC3; Inflammatory; Microglia; NF-κB pathway; STAT1; Spinal cord injury; Valproic acid.
Conflict of interest statement
Ethics approval
The experimental protocols in the present study including all the surgical procedures and animal usages conformed to the guidelines for the care and use of laboratory animals by the National Institutes of Health (NIH) and were approved by the Fujian Medical University Experimental Animal Ethics Committee (Fuzhou, China).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

