Liraglutide Modulates Appetite and Body Weight Through Glucagon-Like Peptide 1 Receptor-Expressing Glutamatergic Neurons
- PMID: 29776968
- PMCID: PMC6054439
- DOI: 10.2337/db17-1385
Liraglutide Modulates Appetite and Body Weight Through Glucagon-Like Peptide 1 Receptor-Expressing Glutamatergic Neurons
Abstract
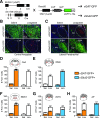
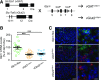
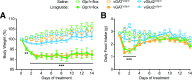
Glucagon-like peptide 1 receptor (GLP-1R) agonists are U.S. Food and Drug Administration-approved weight loss drugs. Despite their widespread use, the sites of action through which GLP-1R agonists (GLP1RAs) affect appetite and body weight are still not fully understood. We determined whether GLP-1Rs in either GABAergic or glutamatergic neurons are necessary for the short- and long-term effects of the GLP1RA liraglutide on food intake, visceral illness, body weight, and neural network activation. We found that mice lacking GLP-1Rs in vGAT-expressing GABAergic neurons responded identically to controls in all parameters measured, whereas deletion of GLP-1Rs in vGlut2-expressing glutamatergic neurons eliminated liraglutide-induced weight loss and visceral illness and severely attenuated its effects on feeding. Concomitantly, deletion of GLP-1Rs from glutamatergic neurons completely abolished the neural network activation observed after liraglutide administration. We conclude that liraglutide activates a dispersed but discrete neural network to mediate its physiological effects and that these effects require GLP-1R expression on glutamatergic but not GABAergic neurons.
© 2018 by the American Diabetes Association.
Figures



References
-
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 - PubMed
-
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:728–742 - PubMed
-
- Pi-Sunyer X, Astrup A, Fujioka K, et al. .; SCALE Obesity and Prediabetes NN8022-1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22 - PubMed
-
- van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol 2014;221:T1–T16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

