Cellular Origin and Functional Relevance of Collagen I Production in the Kidney
- PMID: 29777019
- PMCID: PMC6050926
- DOI: 10.1681/ASN.2018020138
Cellular Origin and Functional Relevance of Collagen I Production in the Kidney
Abstract
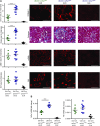
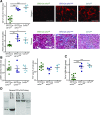
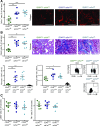
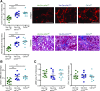
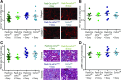
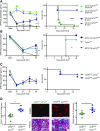
Background Interstitial fibrosis is associated with chronic renal failure. In addition to fibroblasts, bone marrow-derived cells and tubular epithelial cells have the capacity to produce collagen. However, the amount of collagen produced by each of these cell types and the relevance of fibrosis to renal function are unclear.Methods We generated conditional cell type-specific collagen I knockout mice and used (reversible) unilateral ureteral obstruction and adenine-induced nephropathy to study renal fibrosis and function.Results In these mouse models, hematopoietic, bone marrow-derived cells contributed to 38%-50% of the overall deposition of collagen I in the kidney. The influence of fibrosis on renal function was dependent on the type of damage. In unilateral ureteral obstruction, collagen production by resident fibroblasts was essential to preserve renal function, whereas in the chronic model of adenine-induced nephropathy, collagen production was detrimental to renal function.Conclusions Our data show that hematopoietic cells are a major source of collagen and that antifibrotic therapies need to be carefully considered depending on the type of disease and the underlying cause of fibrosis.
Keywords: chronic kidney disease; chronic renal failure; fibrosis; immunology; interstitial fibrosis.
Copyright © 2018 by the American Society of Nephrology.
Figures



References
-
- Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

