CD4 T Cell Affinity Diversity Is Equally Maintained during Acute and Chronic Infection
- PMID: 29777029
- PMCID: PMC6497530
- DOI: 10.4049/jimmunol.1800295
CD4 T Cell Affinity Diversity Is Equally Maintained during Acute and Chronic Infection
Abstract
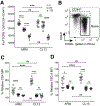
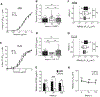
TCR affinity for peptide MHC dictates the functional efficiency of T cells and their propensity to differentiate into effectors and form memory. However, in the context of chronic infections, it is unclear what the overall profile of TCR affinity for Ag is and if it differs from acute infections. Using the comprehensive affinity analysis provided by the two-dimensional micropipette adhesion frequency assay and the common indirect affinity evaluation methods of MHC class II tetramer and functional avidity, we tracked IAb GP61-80-specific cells in the mouse model of acute (Armstrong) and chronic (clone 13) lymphocytic choriomeningitis virus infection. In each response, we show CD4 T cell population affinity peaks at the effector phase and declines with memory. Of interest, the range and average relative two-dimensional affinity was equivalent between acute and chronic infection, indicating chronic Ag exposure did not skew TCR affinity. In contrast, functional and tetramer avidity measurements revealed divergent results and lacked a consistent correlation with TCR affinity. Our findings highlight that the immune system maintains a diverse range in TCR affinity even under the pressures of chronic Ag stimulation.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures




References
-
- Corr M, Slanetz AE, Boyd LF, Jelonek MT, Khilko S, al-Ramadi BK, Kim YS, Maher SE, Bothwell AL, and Margulies DH. 1994. T cell receptor-MHC class I peptide interactions: affinity, kinetics, and specificity. Science 265: 946–949. - PubMed
-
- Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Holländer GA, Gascoigne NR, and Palmer E. 2006. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444: 724–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

