Septin-dependent remodeling of cortical microtubule drives cell reshaping during epithelial wound healing
- PMID: 29777035
- PMCID: PMC6031381
- DOI: 10.1242/jcs.212647
Septin-dependent remodeling of cortical microtubule drives cell reshaping during epithelial wound healing
Abstract
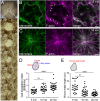
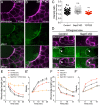
Wounds in embryos heal rapidly through contraction of the wound edges. Despite well-recognized significance of the actomyosin purse string for wound closure, roles for other cytoskeletal components are largely unknown. Here, we report that the septin cytoskeleton cooperates with actomyosin and microtubules to coordinate circumferential contraction of the wound margin and concentric elongation of wound-proximal cells in Xenopus laevis embryos. Microtubules reoriented radially, forming bundles along lateral cell cortices in elongating wound-proximal cells. Depletion of septin 7 (Sept7) slowed wound closure by attenuating the wound edge contraction and cell elongation. ROCK/Rho-kinase inhibitor-mediated suppression of actomyosin contractility enhanced the Sept7 phenotype, whereas the Sept7 depletion did not affect the accumulation of actomyosin at the wound edge. The cortical microtubule bundles were reduced in wound-proximal cells in Sept7 knockdown (Sept7-KD) embryos, but forced bundling of microtubules mediated by the microtubule-stabilizing protein Map7 did not rescue the Sept7-KD phenotype. Nocodazole-mediated microtubule depolymerization enhanced the Sept7-KD phenotype, suggesting that Sept7 is required for microtubule reorganization during cell elongation. Our findings indicate that septins are required for the rapid wound closure by facilitating cortical microtubule reorganization and the concentric elongation of surrounding cells.
Keywords: Cytoskeleton; Embryonic wound healing; Epidermis; Xenopus laevis.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures






References
-
- Ageta-Ishihara N., Miyata T., Ohshima C., Watanabe M., Sato Y., Hamamura Y., Higashiyama T., Mazitschek R., Bito H. and Kinoshita M. (2013). Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat. Commun. 4, 2532 10.1038/ncomms3532 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

