A glial blueprint for gliomagenesis
- PMID: 29777182
- PMCID: PMC6536307
- DOI: 10.1038/s41583-018-0014-3
A glial blueprint for gliomagenesis
Abstract
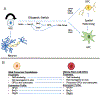
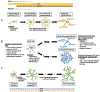
Gliomas are heterogeneous tumours derived from glial cells and remain the deadliest form of brain cancer. Although the glioma stem cell sits at the apex of the cellular hierarchy, how it produces the vast cellular constituency associated with frank glioma remains poorly defined. We explore glioma tumorigenesis through the lens of glial development, starting with the neurogenic-gliogenic switch and progressing through oligodendrocyte and astrocyte differentiation. Beginning with the factors that influence normal glial linage progression and diversity, a pattern emerges that has useful parallels in the development of glioma and may ultimately provide targetable pathways for much-needed new therapeutics.
Figures


References
-
-
Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674, doi:10.1016/j.cell.2011.02.013S0092-8674(11)00127-9 [pii] (2011).
** The expanded version of the canonical primer on cancer biology.
-
-
-
Takebe N et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 12, 445–464, doi:10.1038/nrclinonc.2015.61 (2015).
** A comprehensive review of current pipeline therapies targeting developmental pathway components for glioma treatment.
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

