Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza
- PMID: 29777224
- PMCID: PMC5985949
- DOI: 10.1038/s41590-018-0111-5
Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza
Erratum in
-
Author Correction: Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza.Nat Immunol. 2019 Mar;20(3):373. doi: 10.1038/s41590-019-0328-y. Nat Immunol. 2019. PMID: 30728492
Abstract
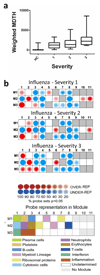
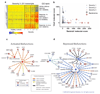
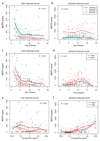
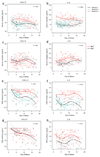
Transcriptional profiles and host-response biomarkers are used increasingly to investigate the severity, subtype and pathogenesis of disease. We now describe whole-blood mRNA signatures and concentrations of local and systemic immunological mediators in 131 adults hospitalized with influenza, from whom extensive clinical and investigational data were obtained by MOSAIC investigators. Signatures reflective of interferon-related antiviral pathways were common up to day 4 of symptoms in patients who did not require mechanical ventilator support; in those who needed mechanical ventilation, an inflammatory, activated-neutrophil and cell-stress or death ('bacterial') pattern was seen, even early in disease. Identifiable bacterial co-infection was not necessary for this 'bacterial' signature but was able to enhance its development while attenuating the early 'viral' signature. Our findings emphasize the importance of timing and severity in the interpretation of host responses to acute viral infection and identify specific patterns of immune-system activation that might enable the development of novel diagnostic and therapeutic tools for severe influenza.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Influenza's signature move.Nat Immunol. 2018 Jun;19(6):518-520. doi: 10.1038/s41590-018-0115-1. Nat Immunol. 2018. PMID: 29777225 No abstract available.
References
-
- Dawood FS, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. The Lancet infectious diseases. 2012;12:687–695. - PubMed
-
- Bautista E, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. The New England journal of medicine. 2010;362:1708–1719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

