The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies
- PMID: 29778200
- PMCID: PMC7117434
- DOI: 10.1016/j.vetmic.2018.04.026
The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies
Abstract
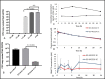
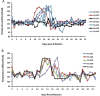
Feline infectious peritonitis (FIP) is a common and highly lethal coronavirus disease of domestic cats. Recent studies of diseases caused by several RNA viruses in people and other species indicate that antiviral therapy may be effective against FIP in cats. The small molecule nucleoside analog GS-441524 is a molecular precursor to a pharmacologically active nucleoside triphosphate molecule. These analogs act as an alternative substrate and RNA-chain terminator of viral RNA dependent RNA polymerase. We determined that GS-441524 was non-toxic in feline cells at concentrations as high as 100 uM and effectively inhibited FIPV replication in cultured CRFK cells and in naturally infected feline peritoneal macrophages at concentrations as low as 1 uM. We determined the pharmacokinetics of GS-441524 in cats in vivo and established a dosage that would sustain effective blood levels for 24 h. In an experimental FIPV infection of cats, GS-441524 treatment caused a rapid reversal of disease signs and return to normality with as little as two weeks of treatment in 10/10 cats and with no apparent toxicity.
Keywords: Cell culture; EC50; Experimental infection; FIP virus (FIPV); Feline infectious peritonitis (FIP); GS-441524; Laboratory cats; Nucleoside analog; Pharmacokinetics; Tri-phosphorylation.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
M. Perron, E. Murakami and Y. Park are employees of Gilead Sciences, Inc., Foster City, CA, USA and hold stock interests in the company.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

