The CF-Sputum Induction Trial (CF-SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial
- PMID: 29778403
- PMCID: PMC5971213
- DOI: 10.1016/S2213-2600(18)30171-1
The CF-Sputum Induction Trial (CF-SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial
Abstract
Background: Pathogen surveillance is challenging but crucial in children with cystic fibrosis-who are often non-productive of sputum even if actively coughing-because infection and lung disease begin early in life. The role of sputum induction as a diagnostic tool for infection has not previously been systematically addressed in young children with cystic fibrosis. We aimed to assess the pathogen yield from sputum induction compared with that from cough swab and single-lobe, two-lobe, and six-lobe bronchoalveolar lavage.
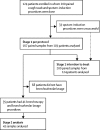
Methods: This prospective internally controlled interventional trial was done at the Children's Hospital for Wales (Cardiff, UK) in children with cystic fibrosis aged between 6 months and 18 years. Samples from cough swab, sputum induction, and single-lobe, two-lobe, and six-lobe bronchoalveolar lavage were matched for within-patient comparisons. Primary outcomes were comparative pathogen yield between sputum induction and cough swab for stage 1, and between sputum induction, and single-lobe, two-lobe, and six-lobe bronchoalveolar lavage for stage 2. Data were analysed as per protocol. This study is registered with the UK Clinical Research Network (14615) and with the International Standard Randomised Controlled Trial Network Registry (12473810).
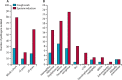
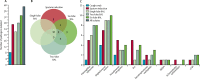
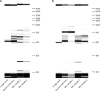
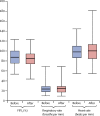
Findings: Between Jan 23, 2012, and July 4, 2017, 124 patients were prospectively recruited to the trial and had 200 sputum induction procedures for stage 1. 167 (84%) procedures were successful and the procedure was well tolerated. Of the 167 paired samples, 63 (38%) sputum-induction samples were pathogen positive compared with 24 (14%) cough swabs (p<0·0001; odds ratio [OR] 7·5; 95% CI 3·19-17·98). More pathogens were isolated from sputum induction than cough swab (79 [92%] of 86 vs 27 [31%] of 86; p<0·0001). For stage 2, 35 patients had a total of 41 paired sputum-induction and bronchoalveolar lavage procedures. Of the 41 paired samples, 28 (68%) were positive for at least one of the concurrent samples. 39 pathogens were isolated. Sputum induction identified 27 (69%) of the 39 pathogens, compared with 22 (56%; p=0·092; OR 3·3, 95% CI 0·91-12·11) on single-lobe, 28 (72%; p=1·0; OR 1·1, 95% CI 0·41-3·15) on two-lobe, and 33 (85%; p=0·21; OR 2·2, 95% CI 0·76-6·33) on six-lobe bronchoalveolar lavage.
Interpretation: Sputum induction is superior to cough swab for pathogen detection, is effective at sampling the lower airway, and is a credible surrogate for bronchoalveolar lavage in symptomatic children. A substantial number of bronchoscopies could be avoided if sputum induction is done first and pathogens are appropriately treated. Both sputum induction and six-lobe bronchoalveolar lavage provide independent, sizeable gains in pathogen detection compared with the current gold-standard two-lobe bronchoalveolar lavage. We propose that sputum induction and six-lobe bronchoalveolar lavage combined are used as standard of care for comprehensive lower airway pathogen detection in children with cystic fibrosis.
Funding: Health and Care Research Wales-Academic Health Science Collaboration and Wellcome Trust Institutional Strategic Support Fund.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Cough swabs less useful but induced sputum very useful in symptomatic older children with cystic fibrosis.Lancet Respir Med. 2018 Jun;6(6):410-411. doi: 10.1016/S2213-2600(18)30183-8. Epub 2018 May 16. Lancet Respir Med. 2018. PMID: 29778402 No abstract available.
-
Six-lobe bronchoalveolar lavage in children with cystic fibrosis - Authors' reply.Lancet Respir Med. 2018 Oct;6(10):e52. doi: 10.1016/S2213-2600(18)30293-5. Epub 2018 Jul 20. Lancet Respir Med. 2018. PMID: 30037712 No abstract available.
-
Six-lobe bronchoalveolar lavage in children with cystic fibrosis.Lancet Respir Med. 2018 Oct;6(10):e51. doi: 10.1016/S2213-2600(18)30295-9. Epub 2018 Jul 20. Lancet Respir Med. 2018. PMID: 30037714 No abstract available.
References
-
- Douglas TA, Brennan S, Gard S. Acquisition and eradication of P aeruginosa in young children with cystic fibrosis. Eur Respir J. 2009;33:305–311. - PubMed
-
- Ramsey KA, Ranganathan S, Park J. Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am J Respir Crit Care Med. 2014;190:1111–1116. - PubMed
-
- Forton J. Induced sputum in young healthy children with cystic fibrosis. Paediatr Respir Rev. 2015;16(suppl 1):6–8. - PubMed
-
- Rosenfeld M, Emerson J, Accurso F. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28:321–328. - PubMed
-
- Brennan S, Gangell C, Wainwright C, Sly PD. Disease surveillance using bronchoalveolar lavage. Paediatr Respir Rev. 2008;9:151–159. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

