Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps
- PMID: 29778504
- PMCID: PMC9057652
- DOI: 10.1016/j.jaci.2018.03.019
Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps
Abstract
Background: IL-25 can function as an early signal for the respiratory type 2 response characteristic of allergic asthma and chronic rhinosinusitis with nasal polyps (CRSwNP). In the mouse gut, tuft cells are the epithelial source of IL-25. However, the source of human airway epithelial IL-25 has remained elusive.
Objective: In this study we sought to determine whether the solitary chemosensory cell (SCC) is the predominant source of IL-25 in the sinonasal epithelium.
Method: Flow cytometry and immunofluorescence for SCCs and IL-25 were used to interrogate polyp and turbinate tissue from patients with CRSwNP. Mucus was collected during acute inflammatory exacerbations from patients with CRSwNP or chronic rhinosinusitis without nasal polyps and IL-25 levels determined by using ELISA. Lastly, sinonasal epithelial cultures derived from polyp and turbinate tissue were stimulated with IL-13 and analyzed for SCC proliferation and IL-25 production.
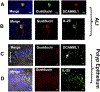
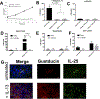
Results: This study demonstrates that a discrete cell type, likely an SCC, characterized by expression of the taste-associated G protein gustducin and the intestinal tuft cell marker doublecortin-like kinase 1, is the predominant source of IL-25 in the human upper airway. Additionally, we show that patients with CRSwNP have increased numbers of SCCs in nasal polyp tissue and that in vitro IL-13 exposure both increased proliferation and induced apical secretion of IL-25 into the mucosal layer.
Conclusions: Inflammatory sinus polyps but not adjacent turbinate tissue show expansion of the SCC population, which is the source of epithelial IL-25.
Keywords: IL-13; IL-25; chronic rhinosinusitis with nasal polyps; doublecortin-like kinase 1; gustducin; solitary chemosensory cells; type 2 inflammation.
Published by Elsevier Inc.
Figures



Comment in
-
Tasting type 2 inflammation in the airways.J Allergy Clin Immunol. 2018 Aug;142(2):403-404. doi: 10.1016/j.jaci.2018.06.009. Epub 2018 Jun 19. J Allergy Clin Immunol. 2018. PMID: 29928925 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

