Replenishable drug depot to combat post-resection cancer recurrence
- PMID: 29779862
- PMCID: PMC6075722
- DOI: 10.1016/j.biomaterials.2018.05.005
Replenishable drug depot to combat post-resection cancer recurrence
Abstract
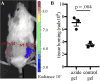
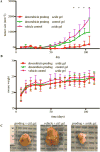
Local drug presentation made possible by drug-eluting depots has demonstrated benefits in a vast array of diseases, including in cancer, microbial infection and in wound healing. However, locally-eluting depots are single-use systems that cannot be refilled or reused after implantation at inaccessible sites, limiting their clinical utility. New strategies to noninvasively refill drug-eluting depots could dramatically enhance their clinical use. In this report we present a refillable hydrogel depot system based on bioorthogonal click chemistry. The click-modified hydrogel depots capture prodrug refills from the blood and subsequently release active drugs locally in a sustained manner. Capture of the systemically-administered refills serves as an efficient and non-toxic method to repeatedly refill depots. Refillable depots in combination with prodrug refills achieve sustained release at precancerous tumor sites to improve cancer therapy while eliminating systemic side effects. The ability to target tissues without enhanced permeability could allow the use of refillable depots in cancer and many other medical applications.
Keywords: Biomaterials; Cancer therapy; Drug delivery; Drug depot; Sustained release.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures




References
-
- Manabe T, Okino H, Maeyama R, Mizumoto K, Nagai E, Tanaka M, Matsuda T. Novel strategic therapeutic approaches for prevention of local recurrence of pancreatic cancer after resection: trans-tissue, sustained local drug-delivery systems. J Control Release. 2004;100:317–330. - PubMed
-
- Indolfi L, Ligorio M, Ting DT, Xega K, Tzafriri AR, Bersani F, Aceto N, Thapar V, Fuchs BC, Deshpande V, Baker AB, Ferrone CR, Haber DA, Langer R, Clark JW, Edelman ER. A tunable delivery platform to provide local chemotherapy for pancreatic ductal adenocarcinoma. Biomaterials. 2016;93:71–82. - PMC - PubMed
-
- Nandi SK, Bandyopadhyay S, Das P, Samanta I, Mukherjee P, Roy S, Kundu B. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol Adv. 2016;34:1305–1317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

