Linear Self-Motion Cues Support the Spatial Distribution and Stability of Hippocampal Place Cells
- PMID: 29779876
- PMCID: PMC5988980
- DOI: 10.1016/j.cub.2018.04.034
Linear Self-Motion Cues Support the Spatial Distribution and Stability of Hippocampal Place Cells
Abstract
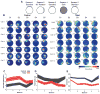
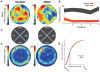
The vestibular system provides a crucial component of place-cell and head-direction cell activity [1-7]. Otolith signals are necessary for head-direction signal stability and associated behavior [8, 9], and the head-direction signal's contribution to parahippocampal spatial representations [10-14] suggests that place cells may also require otolithic information. Here, we demonstrate that self-movement information from the otolith organs is necessary for the development of stable place fields within and across sessions. Place cells in otoconia-deficient tilted mice showed reduced spatial coherence and formed place fields that were located closer to environmental boundaries, relative to those of control mice. These differences reveal an important otolithic contribution to place-cell functioning and provide insight into the cognitive deficits associated with otolith dysfunction.
Keywords: hippocampus; navigation; otolith organs; place cells; vestibular.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. - PubMed
-
- McNaughton BL, Knierim JJ, Wilson MA. The Cognitive Neurosciences. Cambridge, Massachusetts: Massachuetts Institute of Technology; 1995.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

