The Bicarbonate Transporter SLC4A7 Plays a Key Role in Macrophage Phagosome Acidification
- PMID: 29779931
- PMCID: PMC6002608
- DOI: 10.1016/j.chom.2018.04.013
The Bicarbonate Transporter SLC4A7 Plays a Key Role in Macrophage Phagosome Acidification
Abstract
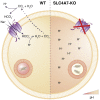
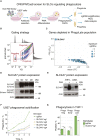
Macrophages represent the first line of immune defense against pathogens, and phagosome acidification is a necessary step in pathogen clearance. Here, we identified the bicarbonate transporter SLC4A7, which is strongly induced upon macrophage differentiation, as critical for phagosome acidification. Loss of SLC4A7 reduced acidification of phagocytosed beads or bacteria and impaired the intracellular microbicidal capacity in human macrophage cell lines. The phenotype was rescued by wild-type SLC4A7, but not by SLC4A7 mutants, affecting transport capacity or cell surface localization. Loss of SLC4A7 resulted in increased cytoplasmic acidification during phagocytosis, suggesting that SLC4A7-mediated, bicarbonate-driven maintenance of cytoplasmic pH is necessary for phagosome acidification. Altogether, we identify SLC4A7 and bicarbonate-driven cytoplasmic pH homeostasis as an important element of phagocytosis and the associated microbicidal functions in macrophages.
Keywords: CRISPR screen; NBC3; NBCn1; SLC4A7; intracellular bacterial killing; macrophages; phagocytosis; phagosome acidification; solute carrier.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells.Circ Res. 2006 Mar 3;98(4):515-23. doi: 10.1161/01.RES.0000204750.04971.76. Epub 2006 Jan 26. Circ Res. 2006. PMID: 16439691
-
The electroneutral Na⁺:HCO₃⁻ cotransporter NBCn1 is a major pHi regulator in murine duodenum.J Physiol. 2012 Jul 15;590(14):3317-33. doi: 10.1113/jphysiol.2011.226506. Epub 2012 May 14. J Physiol. 2012. PMID: 22586225 Free PMC article.
-
The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis.Mol Cell. 2022 Sep 1;82(17):3284-3298.e7. doi: 10.1016/j.molcel.2022.06.008. Epub 2022 Jun 29. Mol Cell. 2022. PMID: 35772404 Free PMC article.
-
The SLC4 family of bicarbonate (HCO₃⁻) transporters.Mol Aspects Med. 2013 Apr-Jun;34(2-3):159-82. doi: 10.1016/j.mam.2012.10.008. Mol Aspects Med. 2013. PMID: 23506864 Free PMC article. Review.
-
Sodium coupled bicarbonate transporters in the kidney, an update.Acta Physiol Scand. 2004 Aug;181(4):505-12. doi: 10.1111/j.1365-201X.2004.01324.x. Acta Physiol Scand. 2004. PMID: 15283764 Review.
Cited by
-
Staphylococcus aureus Uses the GraXRS Regulatory System To Sense and Adapt to the Acidified Phagolysosome in Macrophages.mBio. 2018 Jul 17;9(4):e01143-18. doi: 10.1128/mBio.01143-18. mBio. 2018. PMID: 30018109 Free PMC article.
-
First we eat, then we do everything else: The dynamic metabolic regulation of efferocytosis.Cell Metab. 2021 Nov 2;33(11):2126-2141. doi: 10.1016/j.cmet.2021.08.001. Epub 2021 Aug 24. Cell Metab. 2021. PMID: 34433074 Free PMC article. Review.
-
The transporters SLC35A1 and SLC30A1 play opposite roles in cell survival upon VSV virus infection.Sci Rep. 2019 Jul 18;9(1):10471. doi: 10.1038/s41598-019-46952-9. Sci Rep. 2019. PMID: 31320712 Free PMC article.
-
Potential Theranostic Roles of SLC4 Molecules in Human Diseases.Int J Mol Sci. 2023 Oct 13;24(20):15166. doi: 10.3390/ijms242015166. Int J Mol Sci. 2023. PMID: 37894847 Free PMC article. Review.
-
Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import.Nat Commun. 2020 Dec 1;11(1):6145. doi: 10.1038/s41467-020-19871-x. Nat Commun. 2020. PMID: 33262325 Free PMC article.
References
-
- Arakawa T., Kobayashi-Yurugi T., Alguel Y., Iwanari H., Hatae H., Iwata M., Abe Y., Hino T., Ikeda-Suno C., Kuma H. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science. 2015;350:680–684. - PubMed
-
- Blomen V.A., Majek P., Jae L.T., Bigenzahn J.W., Nieuwenhuis J., Staring J., Sacco R., van Diemen F.R., Olk N., Stukalov A. Gene essentiality and synthetic lethality in haploid human cells. Science. 2015;350:1092–1096. - PubMed
-
- Boedtkjer E., Praetorius J., Matchkov V.V., Stankevicius E., Mogensen S., Füchtbauer A.C., Simonsen U., Füchtbauer E.-M., Aalkjaer C. Disruption of Na+,HCO3− cotransporter NBCn1 (slc4a7) inhibits no-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in miceclinical perspective. Circulation. 2011;124:1819–1829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

