Encoding of Articulatory Kinematic Trajectories in Human Speech Sensorimotor Cortex
- PMID: 29779940
- PMCID: PMC5992088
- DOI: 10.1016/j.neuron.2018.04.031
Encoding of Articulatory Kinematic Trajectories in Human Speech Sensorimotor Cortex
Abstract
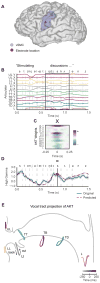
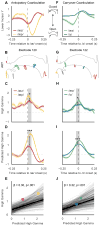
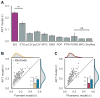
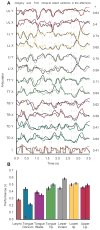
When speaking, we dynamically coordinate movements of our jaw, tongue, lips, and larynx. To investigate the neural mechanisms underlying articulation, we used direct cortical recordings from human sensorimotor cortex while participants spoke natural sentences that included sounds spanning the entire English phonetic inventory. We used deep neural networks to infer speakers' articulator movements from produced speech acoustics. Individual electrodes encoded a diversity of articulatory kinematic trajectories (AKTs), each revealing coordinated articulator movements toward specific vocal tract shapes. AKTs captured a wide range of movement types, yet they could be differentiated by the place of vocal tract constriction. Additionally, AKTs manifested out-and-back trajectories with harmonic oscillator dynamics. While AKTs were functionally stereotyped across different sentences, context-dependent encoding of preceding and following movements during production of the same phoneme demonstrated the cortical representation of coarticulation. Articulatory movements encoded in sensorimotor cortex give rise to the complex kinematics underlying continuous speech production. VIDEO ABSTRACT.
Keywords: articulation; coordination; decoding; electrocorticography; encoding; movement; sensorimotor cortex; speech production; trajectory.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Abbs JH, Gracco VL. Control of complex motor gestures: Orofacial muscles responses to load perturbation of the lip during speech. Journal of Neurophysiology. 1984;51:705–723. - PubMed
-
- Allen MP. Understanding Regression Analysis. Springer; Boston, MA: 1997. Testing hypotheses in nested regression models.
-
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for participants and items. Journal of Memory and Language. 2008;59(4):390–412.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

