Action potential initiation, propagation, and cortical invasion in the hyperdirect pathway during subthalamic deep brain stimulation
- PMID: 29779963
- PMCID: PMC6109410
- DOI: 10.1016/j.brs.2018.05.008
Action potential initiation, propagation, and cortical invasion in the hyperdirect pathway during subthalamic deep brain stimulation
Abstract
Background: High frequency (∼130 Hz) deep brain stimulation (DBS) of the subthalamic region is an established clinical therapy for the treatment of late stage Parkinson's disease (PD). Direct modulation of the hyperdirect pathway, defined as cortical layer V pyramidal neurons that send an axon collateral to the subthalamic nucleus (STN), has emerged as a possible component of the therapeutic mechanisms. However, numerous questions remain to be addressed on the basic biophysics of hyperdirect pathway stimulation.
Objective: Quantify action potential (AP) initiation, propagation, and cortical invasion in hyperdirect neurons during subthalamic stimulation.
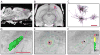
Methods: We developed an anatomically and electrically detailed computational model of hyperdirect neuron stimulation with explicit representation of the stimulating electric field, axonal response, AP propagation, and synaptic transmission.
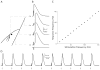
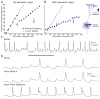
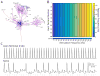
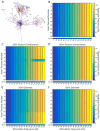
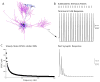
Results: We found robust AP propagation throughout the complex axonal arbor of the hyperdirect neuron. Even at therapeutic DBS frequencies, stimulation induced APs could reach all of the intracortical axon terminals with ∼100% fidelity. The functional result of this high frequency axonal driving of the thousands of synaptic connections made by each directly stimulated hyperdirect neuron is a profound synaptic suppression that would effectively disconnect the neuron from the cortical circuitry.
Conclusions: The synaptic suppression hypothesis integrates the fundamental biophysics of electrical stimulation, axonal transmission, and synaptic physiology to explain a generic mechanism of DBS.
Keywords: Corticofugal axon; Pyramidal neuron; Subthalamic nucleus.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Li Q, Qian ZM, Arbuthnott GW, Ke Y, Yung WH. Cortical effects of deep brain stimulation: implications for pathogenesis and treatment of Parkinson disease. JAMA Neurol. 2014;71(1):100–3. - PubMed
-
- Nambu A, Tokuno H, Inase M, Takada M. Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: comparison with the input zones from the primary motor cortex and the supplementary motor area. Neurosci Lett. 1997;239(1):13–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

