Bimetallic catalysis for C-C and C-X coupling reactions
- PMID: 29780450
- PMCID: PMC5933431
- DOI: 10.1039/c6sc05556g
Bimetallic catalysis for C-C and C-X coupling reactions
Abstract
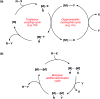
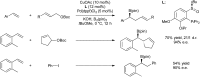
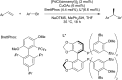
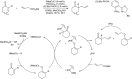
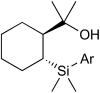
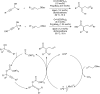
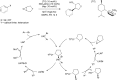
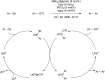
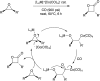
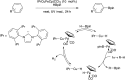
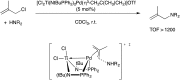
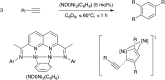
Bimetallic catalysis represents an alternative paradigm for coupling chemistry that complements the more traditional single-site catalysis approach. In this perspective, recent advances in bimetallic systems for catalytic C-C and C-X coupling reactions are reviewed. Behavior which complements that of established single-site catalysts is highlighted. Two major reaction classes are covered. First, generation of catalytic amounts of organometallic species of e.g. Cu, Au, or Ni capable of transmetallation to a Pd co-catalyst (or other traditional cross-coupling catalyst) has allowed important new C-C coupling technologies to emerge. Second, catalytic transformations involving binuclear bond-breaking and/or bond-forming steps, in some cases involving metal-metal bonds, represent a frontier area for C-C and C-X coupling processes.
Figures
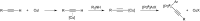
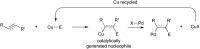






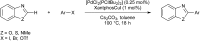





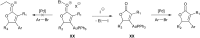


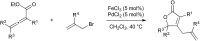
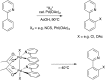






References
-
- Miyaura N., Suzuki A. Chem. Rev. 1995;95:2457–2483.
-
- Heck R. F. Acc. Chem. Res. 1979;12:146–151.
-
- Mkhalid I. A. I., Barnard J. H., Marder T. B., Murphy J. M., Hartwig J. F. Chem. Rev. 2010;110:890–931. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

