Fluorescent dyes and probes for super-resolution microscopy of microtubules and tracheoles in living cells and tissues
- PMID: 29780462
- PMCID: PMC5932598
- DOI: 10.1039/c7sc05334g
Fluorescent dyes and probes for super-resolution microscopy of microtubules and tracheoles in living cells and tissues
Abstract
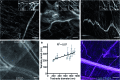
We introduce fluorogenic tubulin probes based on the recently reported fluorescent dyes (510R, 580CP, GeR and SiR) and chemotherapy agents - taxanes (docetaxel, cabazitaxel and larotaxel). The cytotoxicity of the final probe, its staining performance and specificity strongly depend on both components. We found correlation between the aggregation efficiency (related to the spirolactonization of fluorophore) and cytotoxicity. Probe optimization allowed us to reach 29 ± 11 nm resolution in stimulated emission depletion (STED) microscopy images of the microtubule network in living human fibroblasts. Application to living fruit fly (Drosophila melanogaster) tissues highlighted two distinct structures: microtubules and tracheoles. We identified 6-carboxy isomers of 580CP and SiR dyes as markers for chitin-containing taenidia, a component of tracheoles. STED microscopy revealed correlation between the taenidia periodicity and the diameter of the tracheole. Combined tubulin and taenidia STED imaging showed close interaction between the microtubules and respiratory networks in living tissues of the insect larvae.
Figures



References
-
- Grimm J. B., Muthusamy A. K., Liang Y., Brown T. A., Lemon W. C., Patel R., Lu R., Macklin J. J., Keller P. J., Ji N., Lavis L. D. Nat. Methods. 2017;14:987–994. - PMC - PubMed
- Butkevich A. N., Belov V. N., Kolmakov K., Sokolov V. V., Shojaei H., Sidenstein S. C., Kamin D., Matthias J., Vlijm R., Engelhardt J., Hell S. W. Chem.–Eur. J. 2017;23:12114–12119. - PMC - PubMed
- Lukinavičius G., Reymond L., D'Este E., Masharina A., Gottfert F., Ta H., Guther A., Fournier M., Rizzo S., Waldmann H., Blaukopf C., Sommer C., Gerlich D. W., Arndt H. D., Hell S. W., Johnsson K. Nat. Methods. 2014;11:731–733. - PubMed
- Umezawa K., Yoshida M., Kamiya M., Yamasoba T., Urano Y. Nat. Chem. 2017;9:279–286. - PubMed
- Zhang G., Zheng S., Liu H., Chen P. R. Chem. Soc. Rev. 2015;44:3405–3417. - PubMed
-
- Terai T., Nagano T. Pflugers Arch. 2013;465:347–359. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

