Meteoric Metal Chemistry in the Martian Atmosphere
- PMID: 29780678
- PMCID: PMC5947882
- DOI: 10.1002/2017JE005510
Meteoric Metal Chemistry in the Martian Atmosphere
Abstract
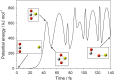
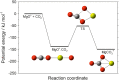
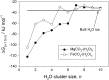
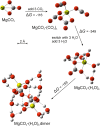
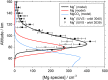
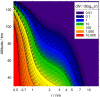
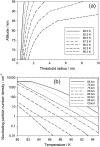
Recent measurements by the Imaging Ultraviolet Spectrograph (IUVS) instrument on NASA's Mars Atmosphere and Volatile EvolutioN mission show that a persistent layer of Mg+ ions occurs around 90 km in the Martian atmosphere but that neutral Mg atoms are not detectable. These observations can be satisfactorily modeled with a global meteoric ablation rate of 0.06 t sol-1, out of a cosmic dust input of 2.7 ± 1.6 t sol-1. The absence of detectable Mg at 90 km requires that at least 50% of the ablating Mg atoms ionize through hyperthermal collisions with CO2 molecules. Dissociative recombination of MgO+.(CO2)n cluster ions with electrons to produce MgCO3 directly, rather than MgO, also avoids a buildup of Mg to detectable levels. The meteoric injection rate of Mg, Fe, and other metals-constrained by the IUVS measurements-enables the production rate of metal carbonate molecules (principally MgCO3 and FeCO3) to be determined. These molecules have very large electric dipole moments (11.6 and 9.2 Debye, respectively) and thus form clusters with up to six H2O molecules at temperatures below 150 K. These clusters should then coagulate efficiently, building up metal carbonate-rich ice particles which can act as nucleating particles for the formation of CO2-ice clouds. Observable mesospheric clouds are predicted to occur between 65 and 80 km at temperatures below 95 K and above 85 km at temperatures about 5 K colder.
Keywords: Mars magnesium layer; Mars mesospheric clouds; ablation; cosmic dust; meteoric metals.
Figures



References
-
- Badnell, D. R. (2006). Radiative recombination data for modeling dynamic finite‐density plasmas. The Astrophysical Journal Supplement Series, 167(2), 334–342. https://doi.org/10.1086/508465 - DOI
-
- Benson, S. W. (1968). Thermochemical kinetics. New York: John Wiley.
-
- Carrillo‐Sánchez, J. D. , Nesvorný, D. , Pokorný, P. , Janches, D. , & Plane, J. M. C. (2016). Sources of cosmic dust in the Earth's atmosphere. Geophysical Research Letters, 43, 11,979–11,986. https://doi.org/10.1002/2016GL071697 - DOI - PMC - PubMed
-
- Clancy, R. T. , Wolff, M. J. , Whitney, B. A. , Cantor, B. A. , & Smith, M. D. (2007). Mars equatorial mesospheric clouds: Global occurrence and physical properties from Mars Global Surveyor Thermal Emission Spectrometer and Mars Orbiter Camera limb observations. Journal of Geophysical Research, 112, E04004 https://doi.org/10.1029/2006JE002805 - DOI
-
- Cox, J. W. , & Dagdigian, P. J. (1984). Chemiluminescence and laser fluorescence study of several mg (1S,3P0) oxidation reactions: On the MgO dissociation energy. The Journal of Physical Chemistry, 88(12), 2455–2459. https://doi.org/10.1021/j150656a008 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
