Altered Cerebellar Short-Term Plasticity but No Change in Postsynaptic AMPA-Type Glutamate Receptors in a Mouse Model of Juvenile Batten Disease
- PMID: 29780879
- PMCID: PMC5956745
- DOI: 10.1523/ENEURO.0387-17.2018
Altered Cerebellar Short-Term Plasticity but No Change in Postsynaptic AMPA-Type Glutamate Receptors in a Mouse Model of Juvenile Batten Disease
Abstract
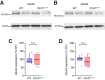
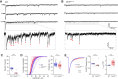
Juvenile Batten disease is the most common progressive neurodegenerative disorder of childhood. It is associated with mutations in the CLN3 gene, causing loss of function of CLN3 protein and degeneration of cerebellar and retinal neurons. It has been proposed that changes in granule cell AMPA-type glutamate receptors (AMPARs) contribute to the cerebellar dysfunction. In this study, we compared AMPAR properties and synaptic transmission in cerebellar granule cells from wild-type and Cln3 knock-out mice. In Cln3Δex1-6 cells, the amplitude of AMPA-evoked whole-cell currents was unchanged. Similarly, we found no change in the amplitude, kinetics, or rectification of synaptic currents evoked by individual quanta, or in their underlying single-channel conductance. We found no change in cerebellar expression of GluA2 or GluA4 protein. By contrast, we observed a reduced number of quantal events following mossy-fiber stimulation in Sr2+, altered short-term plasticity in conditions of reduced extracellular Ca2+, and reduced mossy fiber vesicle number. Thus, while our results suggest early presynaptic changes in the Cln3Δex1-6 mouse model of juvenile Batten disease, they reveal no evidence for altered postsynaptic AMPARs.
Keywords: AMPA receptors; Batten disease; CLN3; EPSCs; cerebellum; short-term plasticity.
Figures




Similar articles
-
Selectively increased sensitivity of cerebellar granule cells to AMPA receptor-mediated excitotoxicity in a mouse model of Batten disease.Neurobiol Dis. 2006 Jun;22(3):575-85. doi: 10.1016/j.nbd.2005.12.018. Epub 2006 Feb 17. Neurobiol Dis. 2006. PMID: 16483786
-
Altered sensitivity of cerebellar granule cells to glutamate receptor overactivation in the Cln3(Δex7/8)-knock-in mouse model of juvenile neuronal ceroid lipofuscinosis.Neurochem Int. 2011 May;58(6):648-55. doi: 10.1016/j.neuint.2011.02.003. Epub 2011 Feb 17. Neurochem Int. 2011. PMID: 21315126 Free PMC article.
-
Age-dependent therapeutic effect of memantine in a mouse model of juvenile Batten disease.Neuropharmacology. 2012 Oct;63(5):769-75. doi: 10.1016/j.neuropharm.2012.05.040. Epub 2012 Jun 6. Neuropharmacology. 2012. PMID: 22683643 Free PMC article.
-
Moving towards therapies for juvenile Batten disease?Exp Neurol. 2008 Jun;211(2):329-31. doi: 10.1016/j.expneurol.2008.02.016. Epub 2008 Mar 4. Exp Neurol. 2008. PMID: 18400221 Review.
-
Localization and processing of CLN3, the protein associated to Batten disease: where is it and what does it do?J Neurosci Res. 2000 Jan 1;59(1):19-23. J Neurosci Res. 2000. PMID: 10658181 Review.
Cited by
-
Early postnatal administration of an AAV9 gene therapy is safe and efficacious in CLN3 disease.Front Genet. 2023 Mar 24;14:1118649. doi: 10.3389/fgene.2023.1118649. eCollection 2023. Front Genet. 2023. PMID: 37035740 Free PMC article.
-
De-palmitoylation by N-(tert-Butyl) hydroxylamine inhibits AMPAR-mediated synaptic transmission via affecting receptor distribution in postsynaptic densities.CNS Neurosci Ther. 2019 Feb;25(2):187-199. doi: 10.1111/cns.12996. Epub 2018 Jun 17. CNS Neurosci Ther. 2019. PMID: 29911316 Free PMC article.
-
Aging, Neurodegenerative Disorders, and Cerebellum.Int J Mol Sci. 2024 Jan 13;25(2):1018. doi: 10.3390/ijms25021018. Int J Mol Sci. 2024. PMID: 38256091 Free PMC article. Review.
-
Modulation of Kv4.2/KChIP3 interaction by the ceroid lipofuscinosis neuronal 3 protein CLN3.J Biol Chem. 2020 Aug 21;295(34):12099-12110. doi: 10.1074/jbc.RA120.013828. Epub 2020 Jul 7. J Biol Chem. 2020. PMID: 32641494 Free PMC article.
References
-
- Autti T, Raininko R, Vanhanen SL, Santavuori P (1996) MRI of neuronal ceroid lipofuscinosis. I. Cranial MRI of 30 patients with juvenile neuronal ceroid lipofuscinosis. Neuroradiology 38:476–482. - PubMed
-
- Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. 10.18637/jss.v067.i01 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
