Circadian and Brain State Modulation of Network Hyperexcitability in Alzheimer's Disease
- PMID: 29780880
- PMCID: PMC5956746
- DOI: 10.1523/ENEURO.0426-17.2018
Circadian and Brain State Modulation of Network Hyperexcitability in Alzheimer's Disease
Abstract
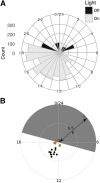
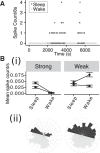
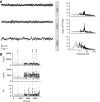
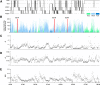
Network hyperexcitability is a feature of Alzheimer' disease (AD) as well as numerous transgenic mouse models of AD. While hyperexcitability in AD patients and AD animal models share certain features, the mechanistic overlap remains to be established. We aimed to identify features of network hyperexcitability in AD models that can be related to epileptiform activity signatures in AD patients. We studied network hyperexcitability in mice expressing amyloid precursor protein (APP) with mutations that cause familial AD, and compared a transgenic model that overexpresses human APP (hAPP) (J20), to a knock-in model expressing APP at physiological levels (APPNL/F). We recorded continuous long-term electrocorticogram (ECoG) activity from mice, and studied modulation by circadian cycle, behavioral, and brain state. We report that while J20s exhibit frequent interictal spikes (IISs), APPNL/F mice do not. In J20 mice, IISs were most prevalent during daylight hours and the circadian modulation was associated with sleep. Further analysis of brain state revealed that IIS in J20s are associated with features of rapid eye movement (REM) sleep. We found no evidence of cholinergic changes that may contribute to IIS-circadian coupling in J20s. In contrast to J20s, intracranial recordings capturing IIS in AD patients demonstrated frequent IIS in non-REM (NREM) sleep. The salient differences in sleep-stage coupling of IIS in APP overexpressing mice and AD patients suggests that different mechanisms may underlie network hyperexcitability in mice and humans. We posit that sleep-stage coupling of IIS should be an important consideration in identifying mouse AD models that most closely recapitulate network hyperexcitability in human AD.
Keywords: Alzheimer’s disease; circadian cycle; epilepsy.
Figures



References
-
- Agostinelli C, Lund U (2013) R package “circular”: circular statistics. Available at https://r-forge.r-project.org/projects/circular.
-
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. 10.18637/jss.v067.i01 - DOI
-
- Bazil CW, Walczak TS (1997) Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia 38:56–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
