Nonmuscle myosin II in cardiac and skeletal muscle cells
- PMID: 29781105
- PMCID: PMC6249054
- DOI: 10.1002/cm.21454
Nonmuscle myosin II in cardiac and skeletal muscle cells
Abstract
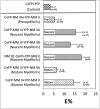
De novo assembly of contractile myofibrils begins with the formation of premyofibrils where filaments of non-muscle myosin (NM II), and actin organize in sarcomeric patterns with Z-Bodies containing muscle-specific alpha-actinin. Interactions of muscle specific myosin (MM II) with NM II occur in a nascent myofibril stage that precedes the assembly of mature myofibrils. By the final stage of myofibrillogenesis, the only myosin II present in the mature myofibrils is MM II. In this current study of myofibril assembly, the three vertebrate isoforms of NM II (A, B, and C) and sarcomeric alpha-actinin, ligated to GFP family proteins, were coexpressed in avian embryonic skeletal and cardiac muscle cells. Each isoform of NM II localized only in the mini-A-Bands of premyofibrils and nascent myofibrils. There was no evidence of localization of NM II in Z-Bodies of premyofibrils and nascent myofibrils or in Z-Bands of mature myofibrils. Fluorescence Recovery After Photobleaching (FRAP) experiments indicated similar exchange rates in premyofibrils for NM II isoforms A and B, whereas the IIC isoform was significantly less dynamic. Fluorescence Resonance Energy Transfer (FRET) measurements of colocalized fluorescent pairs of different NM II isoforms yielded signals similar to identical pairs, indicating copolymerization of the different NM II pairs. The role of NM II may reside in establishing the future sarcomere pattern in mature myofibrils by binding to the oppositely polarized actin filaments that extend between pairs of Z-Bodies along premyofibrils prior to their transformation into mature myofibrils.
Keywords: FRAP; FRET; Myofibrillogenesis; mature myofibril; nascent myofibril; non-muscle myosin II isoforms; premyofibril; sarcomere.
© 2018 Wiley Periodicals, Inc.
Figures







Similar articles
-
STED analysis reveals the organization of nonmuscle muscle II, muscle myosin II, and F-actin in nascent myofibrils.Cytoskeleton (Hoboken). 2022 Dec;79(12):122-132. doi: 10.1002/cm.21729. Epub 2022 Sep 30. Cytoskeleton (Hoboken). 2022. PMID: 36125330
-
A-Band assembly in avian skeletal muscles observed with super-resolution microscopy.Cytoskeleton (Hoboken). 2023 Nov-Dec;80(11-12):461-471. doi: 10.1002/cm.21792. Epub 2023 Sep 28. Cytoskeleton (Hoboken). 2023. PMID: 37767774 Free PMC article.
-
Myofibrillogenesis in skeletal muscle cells in zebrafish.Cell Motil Cytoskeleton. 2009 Aug;66(8):556-66. doi: 10.1002/cm.20365. Cell Motil Cytoskeleton. 2009. PMID: 19382198 Free PMC article.
-
Assembly and Maintenance of Myofibrils in Striated Muscle.Handb Exp Pharmacol. 2017;235:39-75. doi: 10.1007/164_2016_53. Handb Exp Pharmacol. 2017. PMID: 27832381 Review.
-
Myofibrillogenesis in skeletal muscle cells.Clin Orthop Relat Res. 2002 Oct;(403 Suppl):S153-62. doi: 10.1097/00003086-200210001-00018. Clin Orthop Relat Res. 2002. PMID: 12394464 Review.
Cited by
-
Styxl2 regulates de novo sarcomere assembly by binding to non-muscle myosin IIs and promoting their degradation.Elife. 2024 Jun 3;12:RP87434. doi: 10.7554/eLife.87434. Elife. 2024. PMID: 38829202 Free PMC article.
-
Analyses of Off-Target Effects on Cardiac and Skeletal Muscles by Berberine, a Drug Used to Treat Cancers and Induce Weight Loss.Cytoskeleton (Hoboken). 2025 Jun;82(6):344-359. doi: 10.1002/cm.21950. Epub 2024 Nov 11. Cytoskeleton (Hoboken). 2025. PMID: 39526308
-
Fundamental study on structural formation, amino acids and nucleotide-related compounds of cultivated meat from 3D-cultured pig muscle stem cells.Food Sci Biotechnol. 2024 Dec 17;34(2):457-469. doi: 10.1007/s10068-024-01793-9. eCollection 2025 Jan. Food Sci Biotechnol. 2024. PMID: 39944663
-
Inhibitors of the ubiquitin proteasome system block myofibril assembly in cardiomyocytes derived from chick embryos and human pluripotent stem cells.Cytoskeleton (Hoboken). 2021 Oct;78(10-12):461-491. doi: 10.1002/cm.21697. Epub 2022 May 24. Cytoskeleton (Hoboken). 2021. PMID: 35502133 Free PMC article.
-
Identification of crucial circRNAs in skeletal muscle during chicken embryonic development.BMC Genomics. 2022 Apr 28;23(1):330. doi: 10.1186/s12864-022-08588-4. BMC Genomics. 2022. PMID: 35484498 Free PMC article.
References
-
- Dabiri GA, Ayoob JC, Turnacioglu KK, Sanger JM, Sanger JW. Use of green fluorescent proteins linked to cytoskeletal proteins to analyze myofibrillogenesis in living cells. Methods Enzymol. 1999;302:171–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

