Characterization of the Ionic Liquid/Electrode Interfacial Relaxation Processes Under Potential Polarization for Ionic Liquid Amperometric Gas Sensor Method Development
- PMID: 29781608
- PMCID: PMC7192316
- DOI: 10.1021/acssensors.8b00155
Characterization of the Ionic Liquid/Electrode Interfacial Relaxation Processes Under Potential Polarization for Ionic Liquid Amperometric Gas Sensor Method Development
Abstract
Electrochemical amperometric sensors require a constant or varying potential at the working electrode that drives redox reactions of the analyte for detection. The interfacial redox reaction(s) can result in the formation of new chemical products that could change the initial condition of the electrode/electrolyte interface. If the products are not inert and/or cannot be removed from the system such that the initial condition of the electrode/electrolyte interface cannot be restored, the sensor signal baseline would consequently drift, which is problematic for the continuous and real-time sensors. By setting the electrode potential with the periodical ON-OFF mode, electrolysis can be forestalled during the off mode which can minimize the sensor signal baseline drift and reduce the power consumption of the sensor. However, it is known that the relaxation of the structure in the electrical double layer at the ionic liquid/electrode interface to the steps of the electrode potential is slow. This work characterized the electrode/electrolyte interfacial relaxation process of an ionic liquid based electrochemical gas (IL-EG) sensor by performing multiple potential step experiments in which the potential is stepped from an open circuit potential (OCP) to the amperometric sensing potential at various frequencies with different time periods. Our results showed that by shortening the sensing period as well as extending the idle period (i.e., enlarge the ratio of idle period versus sensing period) of the potential step experiments, the electrode/electrolyte interface is prone to relax to its original state, and thus reduces the baseline drift. Additionally, the high viscosity of the ionic liquids is beneficial for electrochemical regeneration via the implementation of a conditioning step at zero volts at the electrode/electrolyte. By setting the working electrode at zero volts instead of OCP, our results showed that it could further minimize the baseline drift, enhance the sensing signal stability, and extend the functioning lifetime of a continuous IL-EG oxygen sensor.
Keywords: amperometric sensor; electric double layer; interfacial relaxation; ionic liquids; oxygen sensor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

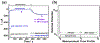



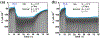

Similar articles
-
Toward continuous amperometric gas sensing in ionic liquids: rationalization of signal drift nature and calibration methods.Anal Bioanal Chem. 2018 Jul;410(19):4587-4596. doi: 10.1007/s00216-018-1090-y. Epub 2018 Jun 13. Anal Bioanal Chem. 2018. PMID: 29947905
-
Ionic liquids as green solvents and electrolytes for robust chemical sensor development.Acc Chem Res. 2012 Oct 16;45(10):1667-77. doi: 10.1021/ar200330v. Epub 2012 Aug 15. Acc Chem Res. 2012. PMID: 22891895
-
Single-Frequency Impedance Studies on an Ionic Liquid-Based Miniaturized Electrochemical Sensor toward Continuous Low-Temperature CO2 Monitoring.ACS Sens. 2023 Jan 27;8(1):197-206. doi: 10.1021/acssensors.2c02040. Epub 2023 Jan 11. ACS Sens. 2023. PMID: 36630698
-
Application of Ionic Liquids in Amperometric Gas Sensors.Crit Rev Anal Chem. 2016;46(2):122-38. doi: 10.1080/10408347.2014.989957. Crit Rev Anal Chem. 2016. PMID: 25830724 Review.
-
Ionic Liquids for Supercapacitor Applications.Top Curr Chem (Cham). 2017 Jun;375(3):63. doi: 10.1007/s41061-017-0150-7. Epub 2017 May 30. Top Curr Chem (Cham). 2017. PMID: 28560657 Review.
References
-
- Räsänen R-M; Nousiainen M; Peräkorpi K; Sillanpää M; Polari L; Anttalainen O; Utriainen M Determination of gas phase triacetone triperoxide with aspiration ion mobility spectrometry and gas chromatography–mass spectrometry. Anal. Chim. Acta 2008, 623 (1), 59–65. - PubMed
-
- Datta S; Rule a M.; Mihalic JN.; Chillrud SN; Bostick BC.; Ramos-Bonilla JP.; Han I; Polyak LM.; Geyh a S.; Breysse PN Use of X-ray Absorption Spectroscopy To Speciate Manganese in Airborne Particulate Matter from Five Counties Across the United States. Environ. Sci. Technol 2012, 46 (6), 3101–3109. - PMC - PubMed
-
- Bruns EA; Perraud V; Greaves J; Finlayson-Pitts BJ Atmospheric solids analysis probe mass spectrometry: a new approach for airborne particle analysis. Anal. Chem 2010, 82 (14), 5922–5927. - PubMed
-
- Stetter JR; Pan L Amperometric carbon monoxide sensor module for residential alarms; US Patent 5,331,310 A, 1994-07-19.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

