Lymphoid tissue fibrosis is associated with impaired vaccine responses
- PMID: 29781814
- PMCID: PMC6025977
- DOI: 10.1172/JCI97377
Lymphoid tissue fibrosis is associated with impaired vaccine responses
Abstract
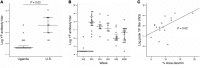
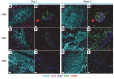
Vaccine responses vary by geographic location. We have previously described how HIV-associated inflammation leads to fibrosis of secondary lymph nodes (LNs) and T cell depletion. We hypothesized that other infections may cause LN inflammation and fibrosis, in a process similar to that seen in HIV infection, which may lead to T cell depletion and affect vaccine responses. We studied LNs of individuals from Kampala, Uganda, before and after yellow fever vaccination (YFV) and found fibrosis in LNs that was similar to that seen in HIV infection. We found blunted antibody responses to YFV that correlated to the amount of LN fibrosis and loss of T cells, including T follicular helper cells. These data suggest that LN fibrosis is not limited to HIV infection and may be associated with impaired immunologic responses to vaccines. This may have an impact on vaccine development, especially for infectious diseases prevalent in the developing world.
Keywords: Adaptive immunity; Bacterial vaccines; Fibrosis; Immunology; Vaccines.
Conflict of interest statement
Figures

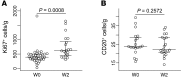

Comment in
-
Lymph node fibrosis: a structural barrier to unleashing effective vaccine immunity.J Clin Invest. 2018 Jul 2;128(7):2743-2745. doi: 10.1172/JCI121053. Epub 2018 May 21. J Clin Invest. 2018. PMID: 29781815 Free PMC article.
References
-
- Hanlon P, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987;1(8546):1342–1345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

