A Microfluidic Platform for Longitudinal Imaging in Caenorhabditis elegans
- PMID: 29782012
- PMCID: PMC6101091
- DOI: 10.3791/57348
A Microfluidic Platform for Longitudinal Imaging in Caenorhabditis elegans
Abstract
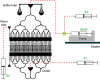
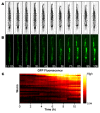
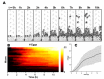
In the last decade, microfluidic techniques have been applied to study small animals, including the nematode Caenorhabditis elegans, and have proved useful as a convenient live imaging platform providing capabilities for precise control of experimental conditions in real time. In this article, we demonstrate live imaging of individual worms employing WormSpa, a previously-published custom microfluidic device. In the device, multiple worms are individually confined to separate chambers, allowing multiplexed longitudinal surveillance of various biological processes. To illustrate the capability, we performed proof-of-principle experiments in which worms were infected in the device with pathogenic bacteria, and the dynamics of expression of immune response genes and egg laying were monitored continuously in individual animals. The simple design and operation of this device make it suitable for users with no previous experience with microfluidic-based experiments. We propose that this approach will be useful for many researchers interested in longitudinal observations of biological processes under well-defined conditions.
References
-
- Lopez-Maury L, Marguerat S, Bahler J. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat Rev Genet. 2008;9(8):583–593. - PubMed
-
- de Nadal E, Ammerer G, Posas F. Controlling gene expression in response to stress. Nat Rev Genet. 2011;12(12):833–845. - PubMed
-
- Hulme SE, Shevkoplyas SS, Samuel A. Microfluidics: Streamlining discovery in worm biology. Nat Methods. 2008;5(7):589–590. - PubMed
-
- Kopito RB, Levine E. Durable spatiotemporal surveillance of Caenorhabditis elegans response to environmental cues. Lab Chip. 2014;14(4):764–770. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources