Use of Most Bothersome Symptom as a Coprimary Endpoint in Migraine Clinical Trials: A Post-Hoc Analysis of the Pivotal ZOTRIP Randomized, Controlled Trial
- PMID: 29782049
- PMCID: PMC6174959
- DOI: 10.1111/head.13327
Use of Most Bothersome Symptom as a Coprimary Endpoint in Migraine Clinical Trials: A Post-Hoc Analysis of the Pivotal ZOTRIP Randomized, Controlled Trial
Abstract
Objective: To better understand the utility of using pain freedom and most bothersome headache-associated symptom (MBS) freedom as co-primary endpoints in clinical trials of acute migraine interventions.
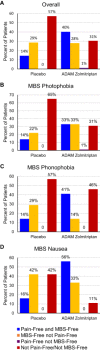
Background: Adhesive dermally applied microarray (ADAM) is an investigational system for intracutaneous drug administration. The recently completed pivotal Phase 2b/3 study (ZOTRIP), evaluating ADAM zolmitriptan for the treatment of acute moderate to severe migraine, was one of the first large studies to incorporate MBS freedom and pain freedom as co-primary endpoints per recently issued guidance by the US Food and Drug Administration. In this trial, the proportion of patients treated with ADAM zolmitriptan 3.8 mg, who were pain-free and MBS-free at 2 hours post-dose, was significantly higher than for placebo.
Methods: We undertook a post-hoc analysis of data from the ZOTRIP trial to examine how the outcomes from this trial compare to what might have been achieved using the conventional co-primary endpoints of pain relief, nausea, photophobia, and phonophobia.
Results: Of the 159 patients treated with ADAM zolmitriptan 3.8 mg or placebo, prospectively designated MBS were photophobia (n = 79), phonophobia (n = 43), and nausea (n = 37). Two-hour pain free rates in those with photophobia as the MBS were 36% for ADAM zolmitriptan 3.8 mg and 14% for placebo (P = .02). Corresponding rates for those with phonophobia as the MBS were 14% and 41% (P = .05). For those whose MBS was nausea, corresponding values were 56% and 16%, respectively (P = .01). Two-hour freedom from the MBS for active drug vs placebo were 67% vs 35% (P < .01) for photophobia, 55% vs 43% (P = .45) for phonophobia, and 89% vs 58% for nausea (P = .04). MBS freedom but not pain freedom was achieved in 28%. Only 1 patient (1%) achieved pain freedom, but not MBS freedom. The proportion with both pain and MBS freedom was highest (56%) among those whose MBS was nausea.
Conclusion: In this study, the use of MBS was feasible and seemed to compare favorably to the previously required 4 co-primary endpoints.
Keywords: adhesive dermally applied microarray; drug delivery; headache; intracutaneous; migraine; triptan; zolmitriptan.
© 2018 The Authors Headache: The Journal of Head and Face Pain published by Wiley Periodicals, Inc. on behalf of American Headache Society.
Figures
References
-
- Migraine: Developing Drugs for Acute Treatment Guidance for Industry. https://www.fda.gov/downloads/drugs/guidances/ucm419465.pdf
-
- Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: Detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633‐658. - PubMed
-
- Martelletti P, Curto M. Headache: Cluster headache treatment ‐ RCTs versus real‐world evidence. Nat Rev Neurol. 2016;12:557‐558. - PubMed
-
- Kellerman DJ, Ameri M, Tepper SJ. Rapid systemic delivery of zolmitriptan using an adhesive dermally applied microarray. Pain Manage. 2017;7:559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical