Exploration of alternative test methods to evaluate phototoxicity of ophthalmic agents by using Statens Seruminstitut Rabbit Cornea cell lines and 3D human reconstituted cornea models
- PMID: 29782497
- PMCID: PMC5962060
- DOI: 10.1371/journal.pone.0196735
Exploration of alternative test methods to evaluate phototoxicity of ophthalmic agents by using Statens Seruminstitut Rabbit Cornea cell lines and 3D human reconstituted cornea models
Abstract
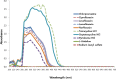
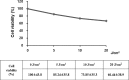
Many chemicals have been reported to induce phototoxicity. The absorbance of light energy within the sunlight range is a common characteristic of phototoxicity. The 3T3 NRU phototoxicity test (PT) in 3T3 mouse skin fibroblasts has been used to identify the phototoxic potential induced by excited chemicals after exposure to ultra violet (UV). However, as phototoxicity may occur in ocular cells, it is necessary to develop a more suitable test for cornea-derived cells. In this study, we attempted to establish a new in vitro PT method in rabbit corneal cell lines (SIRC). We evaluated five ophthalmic agents, ciprofloxacin, levofloxacin, lomefloxacin, norfloxacin, and tetracycline, for their cytotoxic potential and in vitro phototoxicity. The results obtained using 3D human corneal models revealed that the UV-induced eye tissue toxicity by the test substances showed good correlation with those obtained using the in vitro phototoxicity test. However, the results from the 3D PT for ciprofloxacin, norfloxacin, and tetracycline in the 3D human cornea model were only partially comparable. Therefore, we suggest the SIRC cell line as a new phototoxicity test model; however, a sequential testing strategy, such as 3D PT, was also proposed to obtain relevant information for topical eye agents.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Lynch AM, Wilcox P. Review of the performance of the 3T3 NRU in vitro phototoxicity assay in the pharmaceutical industry. Exp Toxicol Pathol. 2011;63(3):209–14. doi: 10.1016/j.etp.2009.12.001 . - DOI - PubMed
-
- Neumann NJ, Blotz A, Wasinska-Kempka G, Rosenbruch M, Lehmann P, Ahr HJ, et al. Evaluation of phototoxic and photoallergic potentials of 13 compounds by different in vitro and in vivo methods. Journal of photochemistry and photobiology B, Biology. 2005;79(1):25–34. doi: 10.1016/j.jphotobiol.2004.11.014 . - DOI - PubMed
-
- Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug safety: an international journal of medical toxicology and drug experience. 2002;25(5):345–72. . - PubMed
-
- Spielmann H, Muller L, Averbeck D, Balls M, Brendler-Schwaab S, Castell JV, et al. The second ECVAM workshop on phototoxicity testing. The report and recommendations of ECVAM workshop 42. Altern Lab Anim. 2000;28(6):777–814. . - PubMed
-
- Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicology and applied pharmacology. 2004;195(3):298–308. doi: 10.1016/j.taap.2003.08.019 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

