Cell Propagation of Cholera Toxin CTA ADP-Ribosylating Factor by Exosome Mediated Transfer
- PMID: 29783743
- PMCID: PMC5983816
- DOI: 10.3390/ijms19051521
Cell Propagation of Cholera Toxin CTA ADP-Ribosylating Factor by Exosome Mediated Transfer
Abstract
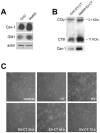
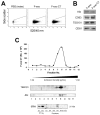
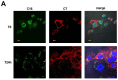
In this study, we report how the cholera toxin (CT) A subunit (CTA), the enzyme moiety responsible for signaling alteration in host cells, enters the exosomal pathway, secretes extracellularly, transmits itself to a cell population. The first evidence for long-term transmission of CT's toxic effect via extracellular vesicles was obtained in Chinese hamster ovary (CHO) cells. To follow the CT intracellular route towards exosome secretion, we used a novel strategy for generating metabolically-labeled fluorescent exosomes that can be counted by flow cytometry assay (FACS) and characterized. Our results clearly show the association of CT with exosomes, together with the heat shock protein 90 (HSP90) and Protein Disulfide Isomerase (PDI) molecules, proteins required for translocation of CTA across the ER membrane into the cytoplasm. Confocal microscopy showed direct internalization of CT containing fluorescent exo into CHO cells coupled with morphological changes in the recipient cells that are characteristic of CT action. Moreover, Me665 cells treated with CT-containing exosomes showed an increase in Adenosine 3',5'-Cyclic Monophosphate (cAMP) level, reaching levels comparable to those seen in cells exposed directly to CT. Our results prompt the idea that CT can exploit an exosome-mediated cell communication pathway to extend its pathophysiological action beyond an initial host cell, into a multitude of cells. This finding could have implications for cholera disease pathogenesis and epidemiology.
Keywords: Caveolin-1; cholera toxin; endocytic pathway; exosomes; monosialganglioside GM1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

